
 |
How it works : Electron |
ELECTRON
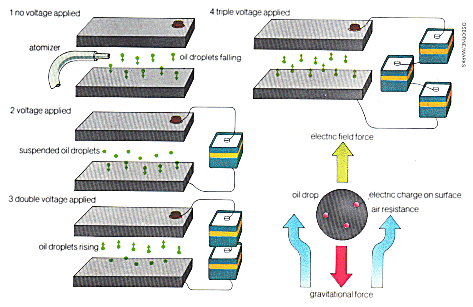
| Below: Millikan's famous oil drop experiment, designed to determine the basic charge of an electron. Minute oil droplets, sprayed from an atomiser between two capacitor plates, acquire a small charge from friction on the nozzle. These drops will move under the force of an electric field applied across the plates and reach a final velocity which is proportional to the strength of the field and also to the electric charge on the oil droplet. Other factors will also influence this velocity such as gravitational force, air resistance and the oils density. At a certain point the electric field will exactly counteract these forces and the drop is suspended. By repeating this experiment many times, a series of values for the charge is reached. These are integral multiples of the charge of a single electron. |
 |
The electron, which is one of the constituent particles of all ATOMS, was discovered at the end of the last century i9 a series of experiments, the most famous being those of the Cambridge physicist JJ Thomson in 1897. He came to the revolutionary conclusion that 'atoms are not indivisible for negatively electrified particles can be torn from them by the action of electrical forces'. This dispelled the belief that the atoms were the basic building blocks of all matter.
Thomson had set up an electric field in an evacuated glass tube and detected something coming from the negatively charged electrode (cathode), travelling towards the positively charged electrode (anode) and lighting up the glass of the tube where it struck. It proved possible to bend these 'cathode rays' with magnetic fields and, in this way, to show that they were negatively charged and lighter than any atom (in fact about one two-thousandth of the mass of the hydrogen atom, which is the lightest of the atoms). The principle and the equipment, in more advanced versions, of Thomson's experiment puts the electron at our service today in the CATHODE RAY TUBES of the television set.
The electrons exist in the atom orbiting around the nucleus. They are held there by the electromagnetic attraction existing between the negative charge carried by the electron and the positive charge of the nucleus, just as the moon is held in orbit by the gravitational attraction between it and the earth. In the heavier atoms as many as ninety electrons can be swirling in a cloud around the nucleus and, in moving from one orbit to another, they are the source of energy which gives us light and X-rays. Also it is the behaviour of the electron clouds of the different atoms and the way the clouds link together (see BOND, CHEMICAL) that gives us the chemical properties of all matter.
The electron is the carrier of ELECTRICITY, passing with comparative ease through the lattice arrangements of atoms in metals. The unit of electric charge carried by a single electron is, however, very small. A million, million, million of them are in the electric current flowing through a 100 watt bulb in a second. Other figures which illustrate the small scale of this particle which plays such an important role in our world are:
the mass of the electron is one ten thousand million million million millionth of a gramme and its radius is one ten million millionth of a centimetre.
One of the revolutionary developments in physics in this century involved the realization that an electron can either behave like a particle or like a wave. As particles they can do such things as knocking silver halide molecules about (thus leaving traces in photographic emulsions) and be accelerated by electric fields. As waves they can form 'diffraction' patterns when passed through foils-like the patterns on the surface of a pond when waves ripple out from dropping in two stones at different places. With the wave-type behaviour it is even possible for the electron to pass through barriers which, looking at the electron as a particle, are impenetrable. This is still one of the puzzles of modern physics.
Reproduced from HOW IT WORKS p873