
 |
How it works : Liquid Crystals |
Liquid crystal materials, materials which though liquid exhibit some of the properties of solid crystal, were a chemical novelty when they were first discovered in about 1889 by H Reinitzer, an Austrian botanist. It is only in recent years that important practical application shave been found for these intriguing materials in the form of liquid crystal displays. These normally take the form of flat a panel of glass which are actually two sheets of glass hermetically, that is water tightly, sealed together with a sandwich of liquid crystal material between them. The display - for example, numbers composed by selecting appropriate bars from a seven bar display - is formed between the two sheets of glass and in one form of display can be viewed by transmitted light while in another form it is viewed by reflected light. A major advantage which liquid crystal displays have over other forms of display is there extremely low power requirement. Another advantage which they have is that they are the only practical electronic display which retains, in fact improves its visibility in direct sunlight. Liquid crystal displays have a disadvantage for some applications in that they cannot be seen in the dark without a power consuming ancillary light source. This is because they do not generate light, but only scatter light falling on or passing through them.
| Below left: some liquid crystals have the property of
changing colour when they are subjected to an increase in temperature. This
photograph shows an example of this type of liquid crystal material undergoing
tests, and the different colour zones are of the liquid at different
temperatures. The blue area at the centre is the hottest and the gradual
decrease in temperature towards the edges is illustrated by the changes in
the colour from blue through green, yellow and orange to red. These liquids
have many potential uses as temperature indicators in industry and
elsewhere. Below right: a typical liquid crystal clock display module. |
 |
Principles and types
Liquid crystals are organic compounds rather similar to oils and with long rod like molecules. In bulk these materials have a cloudy appearance, resembling milk or honey, but when seen as a thin layer sandwiched between two sheets of glass are clear and practically transparent.
There are three main types of liquid crystal material. In all types, the molecules are of an elongated form best visualised as greasy transparent "sausages". These sausages are microscopic in size and it is only the combined effect of many such molecules which produces the observed effects.
In the first type of crystals, smetic, the molecules are the most highly ordered. They form themselves into discrete parallel layers; all the molecules in one layer are parallel to one another, and the molecules in different layers all point the same way.
In the second type, nematic, the molecules arrange themselves with their long axes parallel to one another but take up any position along their axes with respect to adjacent molecules, so that they exhibit a 'grain' but do not form layers.
In the third type, cholesteric, the molecules all point the same way in each layer but each layer is slightly twisted with respect to the ones above and below it so that over large distances a continuous twist is observed to be superimposed upon the parallel arrangement.
All these materials flow like a liquid but exhibit optical properties similar to solid crystals over their working temperature range, typically -5° Celsius to +65° Celsius. The lower temperature is determined by the point at which the material has melted from its solid state and is a liquid, while at the upper temperature limit the liquid loses the its special crystal properties and behaves like an ordinary liquid.
When the liquid crystal material is in its working temperature range it is said to be in the mesophase or anisotropic state. In this condition it has properties similar to those of crystals, in that the light passing through the material from different angles suffers different degrees of refraction or bending of the light rays. Conventional liquids on the other hand are said to be isotropic and these exhibit no such special optical properties.
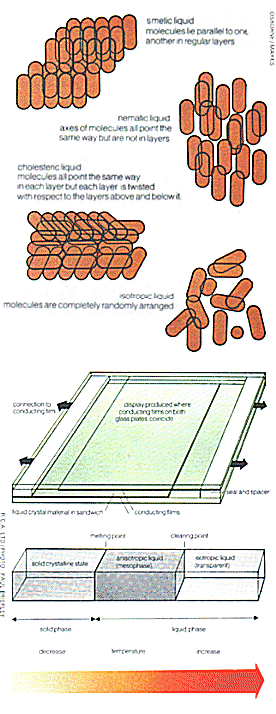 |
| Above: diagrams showing the ways the molecules are arranged in the various types of liquid crystal, the construction of a typical display, and the change of state of the crystals with temperature. |
Operation
An important feature of the molecules of liquid crystal materials is that they possess electrical dipole axes, which are at 90° to the main axes of the molecules.
In a nematic type display at rest is usually arranged by pre-treatment of the glass for the long axes of the molecules to be normal to, that is, standing up on, the glass surfaces. When the operating voltage is applied it has the effect of turning the molecules through a right angle so that the dipole axes are brought into line with the electrical field.
If this was all that happened the liquid in the display would still appear as a clear liquid, as the molecules would still all be lying parallel to one another.
In practice, however, free negative and positive ions, molecules which by gaining or losing an electron have obtained an electrical charge, in the liquid are drawn to the appropriate oppositely charged conducting surfaces and while passing through the liquid locally neutralize the filed across the liquid in the sandwich. This results in small groups of molecules becoming randomly disorientated in a turbulent manner. It is these randomly arranged groups of molecules which because of the anisotropic property scatter light at their interfaces, which have differing refractive indices, to produce what visually appears to be a 'milky' or 'ground glass' effect. This effect produced in a display using nematic liquid is described as a dynamic scattering type of display.
Displays using cholesteric liquids are normally operated on a different principle to that described for nematic liquids. They make use of the fact that the regular twist in the molecule layers causes light passing through the liquid to be twisted. With no applied voltage, polarising filters are placed on either side outside the glass sandwich of the display with their acceptance planes so arranged that light passes through. A small potential is then applied across the parts of the display to be shown of sufficient amplitude twist the molecules through 90° . The light entering through one polarising filter will not now be able to pass out through the polarising filter on the other side and will hence look black to a viewer. Alternatively, if the polarising filters are initially arranged to stop all light, a 90° twist will produce a clear display.
A typical display
A four digit clock display using nomadic liquid might for example, be constructed of seven-bar digits between two glass plates about three inches by one inch (7.5 cm x 2.5 cm). The thickness of the liquid in the sandwich would be about one thousandth of an inch (0.0025 cm) while the glass plates would be about one eighth of an inch thick (0.3 cm) to ensure sufficient rigidity in the glass to maintain the correct gap in the sandwich.
The inside surfaces of the glass plates have deposited on them the pattern which it is desired to be able to display in the form of a transparent conducting layer. This layer is typically tin oxide which has been sintered or baked onto the glass.
Individual connections are made to these conducting areas by, for example, arranging for a row of contact areas along one edge of one of the glass plates so that it can be inserted into a matching contact socket.
The line of conducting material joining the contact area at the edge of the display to the shape to be displayed has to be laid out on the glass plate so that it is not facing any conducting area on the other plate. Only sections to be displayed have matching areas facing one another on opposite glass plates.
Smetic liquids have not yet found favour among display manufacturers but cholesteric liquids are now being used in a similar construction to that described for nomadic liquid except that in this case the display is viewed through polarising filters as described above.
Range of uses
Liquid crystal displays are to be found in portable equipment where they offer a digital readout device consuming only about one thousandth of the power of other common forms of display such as gas discharge or LED light emitting diode) semiconductor.
They are the only form of electronic display which can be easily read in high ambient light levels, even direct sunlight, and in consequence are particularly suited to use in aircraft cockpit and car instrumentation, where the displays must not dazzle the observer at night but must still be clearly visible when sunlight streams in during the daytime. They are also of use in situations requiring intrinsically safe components, such as the mining and chemical industry, for which they are the only form of display device which can offer simultaneously low voltage, low current and low surface temperature, because of their extremely low power dissipation. They can therefore be used in circuits incapable of producing enough energy to initiate an explosion in an explosive atmosphere methane or petrol (gasoline) vapour for example).
Displays are not limited to digital or alphanumeric types but are also available in thermometer or analog form in which successive elements in a row can be activated, or in mimic diagram and dot matrix forms.
Liquid crystal displays are particularly suitable for use with modern complementary metal oxide semiconductor (CMOS) technology (using refined forms of integrated circuits) because their operating voltages and very low power requirements are directly compatible.
Reproduced from HOW IT WORKS p1384