
 |
How it works : Nucleus |
NUCLEUS
Below: most elements have spherical nuclei, but some heavier nuclei are elongated. Two unusual shapes are shown here: those of yttrium (like a flattened disc with projections from the centre of each side) and samarium (like a double doughnut). Both are stable nuclei. This was discovered by 'elastic scattering' bombardment with alpha particles, which are deflected in various directions by large nuclei, giving an indication of the shape of the object they are hitting. |
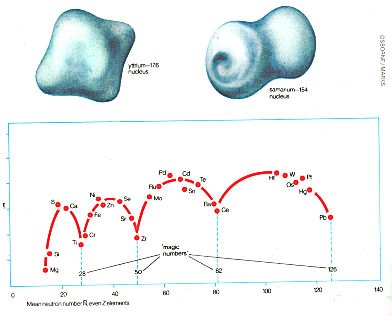 |
Above: physicists have tried to find reasons why the nuclei of some elements are more stable than those of others, and also to build up a system of 'magic numbers' of particles in the nucleus, similar to the numbers of electrons in atoms which make elements stable, and therefore inert, as described in the article on the periodic table. This graph shows the neutron absorption (r,) of nnclei with an even number of protons (even or atomic number). The horizontal scale shows the mean (average) number of neutrons in the nucleus (N). The 'magic numbers', represented by low points in the value of s, ,fall at 28,50,80, and 126. For example, lead (Pb), with a mean neutron number of 126 and atomic number of 50, has ten stable isotopes, more than any other element. Many radioactive elements finally decay to lead. Graphs of other characteristics show similar 'magic numbers'. |
The nucleus is the tiny core of the ATOM where almost all the mass is concentrated. It is built up of PROTONS and NEUTRONS packed together in a volume about a million millionth of a centimetre across, while the atom's ELECTRON cloud spreads out ten thousand times wider.
The number of protons and neutrons in nuclei can vary between one (the single proton at the centre of the HTDROGEN atom) and about 250 (for example, a mixture of 100 protons and 150 neutrons at the centre of the firmium atom). It is the number of protons, equivalent to the number of surrounding electrons, which dictates the type of chemical element, while the number of neutrons grouped with a given number of protons produces several ISOTOPES of the same element. In general the number of neutrons is an excess of the number of protons and the excess is greater in the heavier elements.
Some nuclei are extremely stable. An example is the LEAD nucleus with 82 protons and 126 neutrons; such numbers (and others) became known as 'magic' numbers because the way in which they produce such high stability is not fully understood. Other nuclei are unstable (or radioactive). This is particularly true of the heavy nuclei such as plutonium. They tend to break up either by emitting clumps of two protons and two neutrons (alpha decay) or by emitting electrons as neutrons break up (beta decay). In rare instances, they FISSION into two approximately equal halves.
The discovery that protons and neutrons can cluster together in nuclei was one of the most important ever made in physics because it led to the discovery of a new force Operating in Nature. In the early 1930s, there was no understanding of how positively charged protons could stay so close to one another without repelling, like similar poles of magnets, or of how
neutrons, with no charge at all, could be held. In 1935, a Japanese physicist, H Yukawa, developed the theory that a previously unknown force is in action in the nucleus. It is called the 'strong force' since it is much more powerful than the electrical force which is tending to push the protons apart.
The force operates by the exchange of light particles, known as pions, between the protons and neutrons. These pions cannot be seen when looking at the behaviour of the nucleus as a whole but emerge in large quantities when a nucleus is hit by a high energy particle from an accelerator. The nucleus can be com pared to a group of ball players viewed from a distance. It is not obvious that they are clustered close together because they are exchanging a ball from one to another unless the group is broken up and the ball emerges by itself. The exchange of pions can go on over only very short distances, which explains why the nucleus is packed so close and why the strong force does nor pull in other protons or neutrons from the outskirts of an atom but only captures them when they penetrate the nuclear volume itself.
Knowledge of the major features of the nucleus has made it possible to tap the strong force as a source of energy and to bring nuclear power into the service of mankind.
NYLON MANUFACTURE (see fibre, synthetic)
Reproduced from HOW IT WORKS p1608