 Before
you finish reading this, millions of cells in your body will have killed
themselves - in the interests of keeping you alive. It's something biologists
have only just realised - and it could lead to a medical revolution
Before
you finish reading this, millions of cells in your body will have killed
themselves - in the interests of keeping you alive. It's something biologists
have only just realised - and it could lead to a medical revolution
Nature |
 Before
you finish reading this, millions of cells in your body will have killed
themselves - in the interests of keeping you alive. It's something biologists
have only just realised - and it could lead to a medical revolution
Before
you finish reading this, millions of cells in your body will have killed
themselves - in the interests of keeping you alive. It's something biologists
have only just realised - and it could lead to a medical revolution
You are slicing bread, bleary-eyed, one morning when the knife slips. Your
finger is cut and starts to bleed. You wash it, put on a plaster and head
off for work. Apart from the odd twinge, you think nothing more of it. But
as often happens when you cut yourself, some skin cells have been displaced
down into muscle tissue. If they survive and divide they will grow into tumours.
But there's no need to panic. Your body is taking care of the problem - the
skin cells are self-destructing.
The traditional view of cell development is that cells multiply rapidly before
settling down to specific tasks such as being a blood cell or a brain cell.
But a controversial new theory suggests that the development of all living
things whether humans, animals or plants is shaped largely by the death of
unwanted cells. Death is an intricate part of life, from the very
moment a new organism is created from a single cell.
This startling new view has far- reaching implications: too-rapid cell death
appears to play a key role in many disorders, including heart disease, strokes
and Alzheimer's disease. And mounting evidence suggests that cancer is
the result of cells not dying fast enough - rather than multiplying out
of control as many scientists have previously believed.
|
|
|
|
How cell death can sculpt a foetus Cell suicide helped to shape this three-month-old foetus from a single fertilised cell. The cell divides to form a ball of cells. Some develop,others commit suicide when no longer required |
|
But how do our bodies know which cells to put on the suicide list? It turns
out that all our cells are programmed to die. Immediately. They only
survive because they continually receive biochemical reprieves, telling them
that they may stay alive. If the reprieve doesn't arrive, the cell falls
obediently on its sword.
This programmed self-destruction has been christened apoptosis (the "a" is
pronounced as in ape) by Professor Andrew Wyllie of Edinburgh University
who pioneered the concept of programmed cell death in 1972. The word actually
means "drop out" says Wyllie. "It was used by Greek poets to describe
the dropping of leaves from trees in autumn and I think it conveys
the idea of the loss of cells that ought to die,in the midst of a living
structure.
Martin Raff, professor of biology at University College, London , and another
long-time believer in programmed cell death another name for apoptosis calls
it "death by default".
Raff's experiments have provided strong evidence to support the idea
that the only thing that stops any living cell from putting an end to itself
is the constant receipt of biochemical signal saying "Don't do it! Stay alive!"
Raff took living cells from animals and tried to grow them singly in
culture dishes in the laboratory.The cells all activated their suicide programme
and died,because they were not receiving any "stay alive" messages.
"You need apoptosis in adult life,not just while you are developing as an
embryo," he explains.As an adult you still need to eliminate cells
that have got into the wrong place."
Apoptosis seems to be built into all multi-celled living organisms. Even
the cells of volvox, a microscopic hollow ball of undifferentiated
cells found in plants are programmed to die unless they are told to
live.
Apoptosis is also clearly at work a couple of steps further up the evolutionary
ladder, in the much-studied roundworm.
Scientists have been able to work out exactly where each cell in an adult
worm comes from. This led Professor Robert Horwitz of the Massachusetts Institute
of Technology, America, to investigate exactly what role apoptosis plays
in the sexual development of a kind of roundworm known as Caenorhabditis
elegans, or C elegans for short.
"The two sexes of C. elegans are quite distinct in many ways," says
Horwitz. " But the pattern of cell division that gives rise to them is the
same in both. What makes the difference is apoptosis." For example, the female
develops the cells that will be needed for egg laying, while the equivalent
cells in the male undergo apoptosis rather than development.
Apoptosis is such a fundamental process that the genes controlling it in
roundworms are also the ones that control it in humans - and probably in
all other living things. The search for genes common to different species
has been central to apoptosis research.
The discoverer |
||
"The whole process is as natural as the shedding of leaves in autumn" |
|
|
| Andrew Wyllie (above),
John Kerr and Alistair Currie realised over 20 years ago that cell suicide
was as natural as the shedding of leaves. They saw it as the opposite
of cell division.Cell suicide and cell division keep the number of cells
in an organism in balance. Cell suicide - apoptosis - is different from necrosis, which is the "murder" of cells by external factors like poisons,burning or lack of oxygen. In necrosis the cells disintegrate,often causing inflammation. But in apoptosis,the cell shrinks and its contents are packaged neatly by enzymes, ready for digestion by cells that roam the body cleaning up. But it has taken many years for scientists to appreciate Wyllie's insights. |
||
A human gene called bcl-2 is known to be one of several that can cause human cancer. It blocks off apoptosis so that cells don't self-destruct when they should, and instead grow into tumours. Horwitz found that bcl-2 is very similar to a gene in C. elegans called ced-9, which also protects against cell death. When the human gene is put into the roundworm, it has exactly the same effect as the worm's own ced-9.
For medical researchers, the universal nature of apoptosis is excellent news
because it means that the things they discover about lower animals can also
be applied to humans. It's important news, too, because apoptosis is now
the hottest area of cancer research. The discovery that cells only stay alive
because they constantly receive signals telling them to do so suggests that
tumours may grow from cells that receive a reprieve when they shouldn't,
or that have forgotten how to die.
Dr Gerard Evan and his Colleagues, at the Imperial Cancer Research Fund's (ICRF) laboratories in London, have discovered more about the self-destruct programme and the reprieve signal. Whenever a cell divides, it is the same gene known as c-Myc - that both orders it to divide and, simultaneously , orders it to kill itself.Without the reprieve signal, the cell self-destructs.
But why should cells want to kill themselves every time time they divide?
Evan believes that cell self-destruction is an organism's way of safeguarding
itself. The most dangerous time for the body is when cells divide: if just
one out of all our billions of cells become locked into division, the result
is a tumour. Yet, paradoxically cells must divide if they are to build and
maintain the body.
Evan compares the suicide and reprieve controls acting on c-Myc to the dual-key
system on a nuclear missile. "The keys come in the form of chemical signals,"
he says. One key turns on cell growth but at the same time switches on the
sequence leading to self-destruction. The second key overrides the self-destruct
key. So you must have both keys."
Deciding the fate of a cell: go forth and multiply,or die |
|
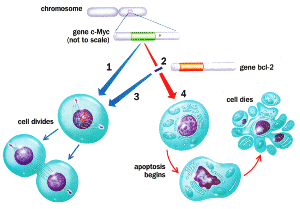 |
The gene c-Myc "decides" whether a cell is going to divide (1) or die(2).But the gene does not act alone; it is part of a complex network of genes and proteins that take part in decision making. Even if c-Myc condemns a cell, bci-2 may step in with a reprieve (3), allowing the cell to go ahead and divide. If it does not,the cell undergoes apoptosis (4) - it commits suicide. |
Liz Harrington, another member of the ICRF team, says: '"Death is built into
the very mechanism by which cells divide. You can't get cancer by merely
acquiring a mutation that makes your cells divide more rapidly, because we
now know that the faster cells divide, the more they die. So a cancer cell
must also acquire a mutation in the machinery that controls cell death. In
the past, cancer researchers have concentrated almost entirely on cell division
and regarded cell death as a curiosity. As a result we've largely ignored
half the picture of what makes a cell cancerous."
This opens up a whole new vista of possibilities for the treatment of cancer.
The c-Myc gene is damaged in most - perhaps all - cancer cells. When
it is damaged it never stops giving the order to divide and it also prevents
cells responding to orders that tell them to stop dividing. But because the
altered c-Myc can still give the suicide signal, the potentially dangerous
cells usually kill themselves and no tumour develops. However, things go
wrong if some other signal blocks the suicide signal and keeps the endlessly
dividing cells alive.
The ICRF team believe that these interfering signals are the hormones upon
which many cancers depend for their survival. If these hormones could be
blocked - perhaps only temporarily - then cancer cells, lacking hormonal
protection, would have to listen to the unwelcome message:
'"Your time's up. Goodbye." Once
the tumour had killed itself off, the hormone-blocking drug could then be
discontinued to minimise any possible side-effects.
In July, the ICRF signed a research agreement with the company Apoptosis
Technology subsidiary of the biotech company Immunogen, to develop drugs
that will, hopefully, block tumour-protecting hormones and make cancers kill
themselves off.
New treatment that turns our bodies' own weaponry against cancer cells |
||||
| The traditional treatment of cancer consists of attacking
the tumour - with surgery, radiation or drugs. But cancer cells may
only be damaged rather than killed if the genes that control apoptosis are
not working properly,which is often the case in cancer. The wounded cells may linger, perhaps even multiplying and making the cancer worse.Anti-cancer drugs and radiation treatment might even prevent apoptosis by knocking out genes, like p53,which ensure that damaged cells commit suicide. This might explain why some cancers become resistant to chemotherapy, and a few actually get more aggressive during treatment. |
|
|||
|
Three new companies,based in the
US, are looking at ways of opening up apoptosis pathways in cancer by modifying
the actions of the genes and proteins involved. Genta, a Californian company,has found new drugs that block bci-2 genes, which allow cancer cells to divide.In a test tube these drugs kill leukemia cells. |
|||
But that's only one of the new approaches to cancer therapy opened up by
the discovery of the role of apoptosis. Dr David Lane of Dundee University
is studying a gene called p53, which has the grim task of ensuring cells
that are becoming malignant do the decent thing and sacrifice themselves
for the common good.
"I call p53 the guardian of genome," says Lane. "When it recognises genetic
damage - the kind of damage that leads to a cell becoming malignant
- it either orders the cell to stop dividing till the damage is repaired
or,if things have gone too far orders to commit suicide by apoptosis.
Gene p53 is rather like adjutant of a Victorian regiment, handing the disgraced
officer a loaded revolver and leaving him alone in the Mess. Suicide will
save the regiment from disgrace and the body from cancer.
The p53 gene is an important guardian. Between a third and a half of all
cancers are thought to result from a mutation that damages p53 so the gene
stops working. If further genetic damage pushes the cell towards malignancy,
there's nothing to stop it if p53 is out of action. And it only has to happen
once in one cell to start a cancer. But now there's hope of a reversal
process.
"What we'd love to do is to get p53 working again in cancer cells," says
Lane." One way is to put healthy p53 genes into cancer cells to compensate
for the defective genes. It works wonderfully in a test tube.So instead
we've been trying to find ways to combat the abnormal protein
produced by the defective p53 gene. It's the proteins produced by genes,
not the genes, that do - or don't do the job. To our great excitement we've
recently started to make the abnormal protein behave like a normal one."
Pharmaceutical companies are working with Lane to develop drugs that could
do the same thing. The aim is to make the cancer cells behave like normal
cells or, if they are too far gone, exterminate themselves. If this idea
works, Lane believes that drugs will be tested within five years. "If we
don't get something by then, I suspect we won't get it at all."
|
Incriminating a gene The brain tumour revealed by
a scan (below) may result from a faulty p53 gene. Recent experiments have
shown that the p53 gene is damaged in most human cancers. |
|
|
| The stark choices facing a damaged cell The gene p53 gives a damaged cell two choices. If the damage is limited, the cell gets time for repair and then divides normally (1). If the damage is not worth repairing, the cell is ordered to undergo apoptosis (2). If p53 is not working properly, the damaged cell goes straight into cell division, perhaps leading to a tumour. | |
Understanding apoptosis may also lead to successful treatments for many diseases,
including strokes, immune system disorders, heart attacks and Alzheimer's
disease. In these conditions much - maybe most - of the damage is caused
not by infection or lack of oxygen, but by cells not receiving the "stay
alive" signals they need to avoid self-destruction. Drugs that are intended
to restore these vital signals before too many brain cells have completely
destroyed themselves are already being tested as a treatment for stroke
victims.
The discovery of apoptosis may precipitate as big a revolution in medicine
as it already has in basic biology.
John Newell