
 |
How it works : Bond,Chemical |
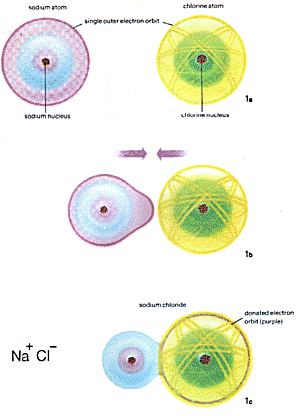 |
| These diagrams represent various forms of chemical bonding commonly found. 1a, an atom of sodium and one of chlorine. 1b, if a solution of sodium hydroxide is mixed with dilute hydrochloric acid the sodium and chlorine ions combine by forming an electrovalent bond to give a molecule of NaCl (1c). |
A chemical bond is the link which joins two ATOMS. The atoms may be identical as in hydrogen gas (H2), or different as in the COMPOUND magnesium oxide (MgO). There are also many chemical compounds made up from more than two atoms. Each atom is joined to another by a bond to form a MOLECULE, which is the smallest particle of a compound that has the properties of that compound. In a molecule of common salt, one atom of sodium (a reactive alkali metal, symbol Na) is bonded to one atom of chlorine (a gas, symbol Cl) to give the white crystalline solid sodium chloride (NaCl). In chloroform one atom of carbon, a solid, is bonded to one atom of hydrogen gas and to three atoms of chlorine to give the liquid chloroform (CHCl3). In the NaCl molecule there is only one chemical bond but in the CHCl3 molecule there are four chemical bonds.
Atoms only form bonds with certain other atoms. This depends on the number of electrons in the atoms concerned and on how these are arranged around the nucleus. The general rule is to form outer stable shells containing a fixed number of electrons which for most common atoms is eight. Hydrogen (H) is the exception, as it requires only two electrons. The number of bonds which an atom can form is called its VALENCY.
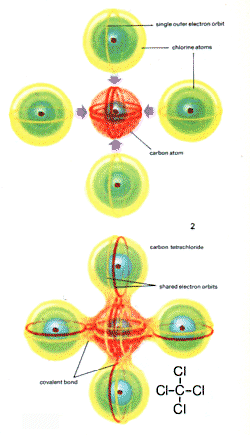 |
| 2, in carbon tetrachloride the four chlorine atoms are bonded in four covalent linkages to the carbon atom |
During a chemical reaction bonds are broken and new bonds are formed. Chemical bonds are formed in three ways:
by sharing an electron pair between two atoms, each atom supplying one of the electrons, which is called a covalent bond; by transferring an electron from one atom to another, which is called an electrovalent bond; and by one atom donating an electron pair to another-this is known as a dative bond.
To show how atoms are bonded in a molecule chemists use structural formulae (see diagrams). Electrovalent bonds are usually formed by inorganic compounds. A sodium atom has one electron in its outer shell which it can fairly readily lose to become a positive sodium ion Na+. Chlorine, however, has seven electrons in its outer shell and so has a tendency to gain another to form the stable number of eight electrons. In so doing it also gains an extra negative charge, becoming a negative ion Cl- Before bonding each atom was electrically neutral, the total positive charge on the nucleus being balanced by the number of electrons, each with a unit of negative charge, which surround the nucleus.
Covalent bonds are the type that exist predominantly in organic compounds such as chloroform (CHCl3), and methane (CH4). In the gas chlorine (Cl2) the molecules are also joined by covalent bonds. When electrons are shared like this the atoms do not form oppositely charged parts.
A dative bond occurs in nitric acid (HNO3). This molecule contains one atom of nitrogen (N) bonded to three atoms of oxygen (O) and one hydrogen atom (H) bonded to one of the oxygen atoms. In pure nitric acid the nitrogen atom donates two electrons to one of the oxygen atoms forming a dative bond with it.
This molecule also contains a double covalent bond between one of the oxygen atoms and the nitrogen atom. Some atoms can form single, double or triple bonds and carbon is the most remarkable example of this facility. It is because carbon atoms can form these bonds and can also bond together to form long chains that there exist today over a million different kinds of molecule containing carbon atoms bonded to each other and to other atoms. Such molecules are the basis of living matter and of the modern chemical industry producing fuels, plastics and drugs.
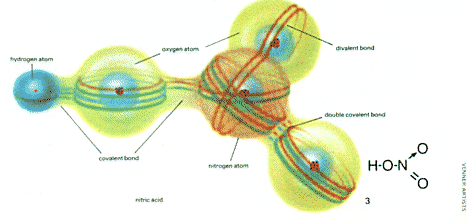 |
| 3, nitric acid has a dative bond, represented by an arrow, between one oxygen and the nitrogen atom. |
In molecules which contain hydrogen atoms and either oxygen or nitrogen atoms another type of bonding occurs, known as hydrogen bonding. These are weak bonds but they are important. Both oxygen and nitrogen have lone pairs of electrons in their outermost shell and the hydrogen atom, which is small and has the positive nucleus near to the surface, tends to 'sit' on the lone electrons -this causes association of molecules. Water, H2O, has molecules formed in this way, and the closeness of the association increases the density of the compound, so that water is a liquid at room temperature and not a gas. Hydrogen bonding is also important in determining the shape of protein molecules, which consist of long coiled chains.
The length of a chemical bond, which is the distance between the nuclei of the bonded atoms, can be determined accurately by X-ray CRYSTALLOGRAPHY. The strength of a chemical bond can also be determined experimentally by measuring the energy changes accompanying the chemical reactions in which the bond is either broken or made.
Reproduced from HOW IT WORKS p308