
 |
How it works : Atom |
The word atom is derived from the Greek word atomos which means 'indivisible', and was used by Democritus as long ago as 400 BC to describe the basic units from which all matter is built up. The 19th century chemists identified about 90 different types of atom, and the total now stands at 105. Atoms can combine in various ways to give all the known chemical COMPOUNDS. The different types, known as the ELEMENTS, can be classified according to similarities in their behaviour in what is known as the PERIODIC TABLE of elements.
In 1897 J J Thomson showed that tiny particles (now known as ELECTRONS) can be torn from atoms by electrical fields. Since the electron is about 2000 times lighter than the atom of hydrogen, which is the lightest of the atoms, the concept of the atom as the indivisible building block of matter fell apart. Further insight came in 1912 from Rutherford's experiment in which high energy particles moving at nearly the speed of light were fired at a thin gold foil. They were expected to brush through the foil with little deviation from their original paths, but some of them flew back in the direction from which they came.
From the way the particles bounced off it was possible to deduce that the atom consists of a very small solid core, known as the NUCLEUS, about a millionth of a millionth of a centimetre across, surrounded by a cloud of electrons giving a total diameter of about a hundredth of a millionth of a centimetre. Most of the atom is 'empty space'- blown up in scale, the nucleus would sit like an orange in the centre of a blurred sphere of elcectrons about the diameter of the Albert Hall or the dome of the Capitol. And yet the minute nucleus contains virtually the total mass of the atom.
The mechanism of the atom became clear soon afterwards. Each electron carries a negative electric charge balanced by the positive charge of a much heavier particle, the PROTON, which is in the nucleus. The electromagnetic attraction between these opposite electric charges holds the electrons in orbit around the nucleus just as gravitational attraction holds the planets in orbit around the sun. The complex QUANTUM theory, one of the most advanced of modern physics, is needed to understand the cloud of electrons and how the different atoms can be built up. In brief, it says that the electrons cannot exist in any orbit around the nucleus, but only in particular ones. The positions electrons can take up are usually referred to as energy states. Only one electron can occupy a particular energy state. If another electron is added it has to go somewhere else around the nucleus.
From these ideas all the atoms known to the chemists can be built up, beginning with the hydrogen atom where a single proton in the nucleus holds a single electron in orbit. In the next smallest, the helium atom, two protons hold two electrons in two slightly different energy states (the helium atom also has two other particles in its nucleus NEU'TRONS which are identical with the proton but which carry no electric charge). And so on to uranium, the heaviest atom found in nature, which packs 92 protons and over 140 neutrons in its nucleus surrounded by a cloud of 92 electrons.
 |
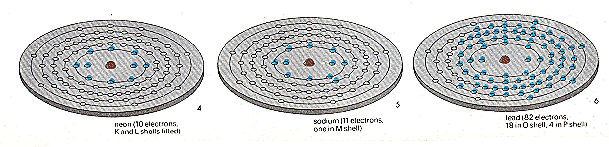 |
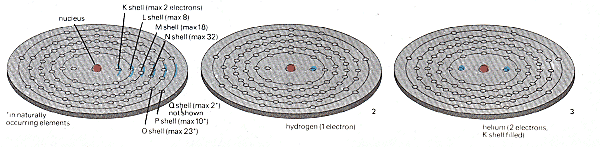
Above, picture 1: this shows the maximum number of spaces, for electrons in shells K to P, there is also a Q shell (maximum 2) found in a few heavy atoms. The simplest atom, hydrogen (picture 2) has only one electron. Helium has two (picture 3). This fills the K shell. Atoms with completely filled shells only are inert - they do not react to form compounds. Helium is an inert gas; so is neon (picture 4), where the L shell is filled too. But sodium (picture 5) has one more electron than neon, and this is forced into the M shell so that sodium is violently' reactive. Picture 6 shows an atom of lead with 82 electrons.
|
When two electrons are in orbit (helium atom) or ten electrons are in orbit (neon atom), for example, the atoms are very stable and do not combine easily with other atoms. On the other band eleven electrons (sodium atom) and seventeen electrons (chlorine atom) are not such stable systems and will readily combine to form a molecule of salt (sodium chloride), which with twenty-eight electrons is more stable. It is the extent to which the electron orbits are filled that explains the similarities in behaviour which led to the classification of atoms in the periodic table.
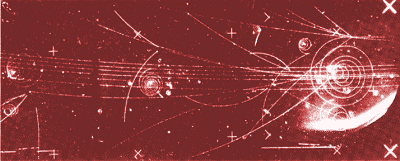 |
| Stable sub-atomic particles such as the electron can be 'seen' when recorded in one of the many thousands of three dimensional photographs taken during an experiment on atoms in a bubble chamber-a device used by nuclear physicists. Here the clearly shown spirals are electrons, which can be knocked out of th atoms in the bubble chamber liquid (usually hydrogen) by incoming high energy particles which are moving near the speed of light. |
The uranium atom is about 240 times heavier than the hydrogen atom, but even heavier ones have been produced artificially by firing additional protons and neutrons into heavy nuclei using PARTICLE ACCELERATORS. The heaviest so far, called hahnium, has 105 protons in its nucleus.
The opposite configuration of an atom, where heavy negative particles (antiprotons) in the nucleus hold light positive particles (antielectrons or positrons) in orbit around them, is equally feasible according to the known laws of physics. Antimatter of this type has in fact been produced in minute quantities by particle accelerators, even as far as producing antinuclei of helium. Antimatter is not found in abundance in the solar system- fortunately, because on encountering normal matter the two types annihilate each other in a burst of energy.
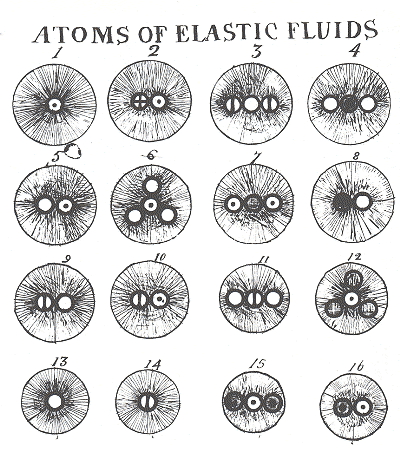 |
| Above:early in the 19th century John Dalton introduced his fanmous atomic theory - chemical elements were made up of tiny particles or atoms of which there was only one kind for each element. He used the symbols for these atoms - a circle with a dot in the centre for hydrogen (1), a circle for oxygen (13),a circle with a cross in it for sulphur (2),and one with a vertical line across it for nitrogen (14). Elastic fluids was the name forthe vapour state of a substance,for example steam (5) or a gas such as hydrogen sulphide (2). |
Understanding the mechanism of the atom explains, in principle, the whole of chemistry. It explains the structure of crystals, the phenomena connected with LIGHT and x-RAYS (absorbed and emitted as electrons jump from one energy level to another) and the whole field of ELECTRONICS. It even underlies the understanding of life itself, since it dictates the molecular structures which allow living cells to function and reproduce. Few recent scientific discoveries in any area could have been made without the help of atomic physics.
Reproduced from HOW IT WORKS p180