
 |
How it works : ELEMENT, chemical |
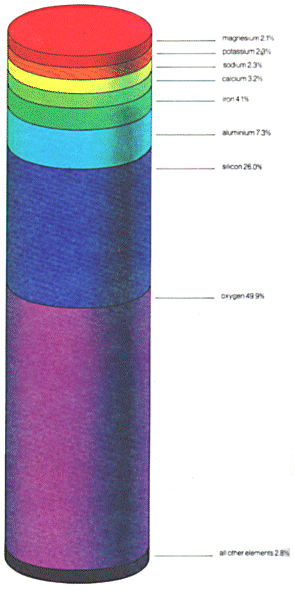
| Below: If it were possible to break down all matter found on the Earth into its constituent elements their distribution would be as represented below By far the most abundant elements are oxygen and silicon which occur widely in various rocks and minerals. On the other hand, the Universe itseif consists of about 90% hydrogen atoms. |
 |
A chemical element is the simplest form of matter; about 90 of them are found in Nature, and are numbered according to the number of electrons in their atoms. The heaviest element which is found in worthwhile amounts is uranium (atomic number 92), but in 1972 traces of plutonium (atomic number 94) were discovered. Still heavier ones can be created artificially at PARTICLE ACCELERATORS by firing subatomic particles (protons and neutrons) at a heavy element. Some of these particles can stick, at least for a short time, and chemical elements as high as atomic number 105 have been observed. The elements that concern us, however, are not thc synthesizcd radioactive ones but those that form the building blocks of all substances. Some elements-usually the fairly inert ones such as gold, platinum, copper, nitrogen-occur free in Nature, hut the majority are combined with other elements to form (sometimes highly complex) chemical COMPOUNDS or mixtures of compounds.
An element is in fact made up of the same type of atoms. So a bar of a pure metal, say the element copper, would consist of millions of individual copper atoms.
As long ago as the 6th century BC, the Greek philosophers developed theories of matter in terms of primary 'elements':
water, air, fire and earth. It was believed that all known substances could be formed from these 'elements', either individually or together. It was BOYLE who coined the word element for simple, pure substances. Then in 1803 DALTON published the results of his investigations into the way elements combined in a 'law of chemical composition'. It was therefore possible to find out the relative weights of atoms from the proportions in which they combined in chemical compounds. For instance he found that eight parts by weight of oxygen and one part by weight of hydrogen were needed to produce water. Subsequently it was found that water is formed from two hydrogen atoms and one of oxygen so that the atomic weight of oxygen is 16 times that of water.
The chemical elements can he distinguished by their atomic mass, the quantity of matter contained in the respective atoms. For example, the lightest of the atomic masses is that of hydrogen with a mass of just over a million, million, million millionth (10-24) of a gramme. These are rather clumsy units, however, and it is easier to use their atomic weight which is a number comparing the relative weight with that of the most commonly found ISOTOPE of carbon. (Isotopes of any particular atom have a different number of neutrons in the nucleus but chemically this makes no difference.) The atomic weight of carbon is taken as 12 precisely and the other elements are hydrogen, 1.008; helium, 4.903; oxygen, 15.999; uranium, 238.03 and so on. (Until 1961 atomic weights were based on taking the oxygen atom as 16 rather than carbon as 12.)
Another important figure is their atomic number This corresponds to the number of protons in the nucleus of the atom. Thus hydrogen is 1, helium 2, oxygen 8, uranium 92 and so on. The atomic number also corresponds to the number of electrons in orbit around the nucleus of the atom, and it is the electrons which dictate all the chemical behaviour of an element.
Classification
Out of the total of 100 or so elements about 65 are classified as metals,
15 as non-metals and 6 as INERT GASES. The remainder are not easy to place;
these include arsenic, germanium and antimony. Fortunately the chemical behaviour
of these elements is not random. Distinct similarities exist such as the
group of ALKALI METALS, namely,lithium, sodium, potassium, rubidium and caesium
and with other groups such as the HALOGENS (fluorine, chlorine, bromine,
and iodine). When all the elements are listed in a table in order of atomic
number, elements with similar characteristics (belonging to the same group)
fall at regular intervals. The table is therefore known as the
PERIODIC TABLE.
ELEMENTARY PARTICLE (see particle physics) _
Reproduced from HOW IT WORKS p890