
 |
How it works : Carbon |
The element carbon occurs in over a million known compounds and only hydrogen is found in a greater number. The chemistry of carbon is so extensive that a whole branch of chemistry, ORGANIC CHEMISTRY, is devoted to it. Carbon, chemical symbol C, is classified as part of group 4b of the PERIODIC TABLE of elements. The other members of this group are SILICON, germanium, TIN and LEAD.
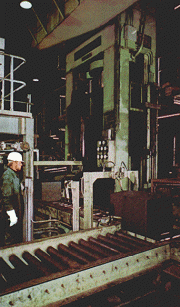
Below: carbon is a good conductor of electricity, which makes it a useful material for electrodes. These large carbon blocks are used for aluminium smelting, which is an electrolytic process. The blocks, made of pitch and coke, are pressed and baked under controlled temperature. |
 |
Occurrence
It is surprising, considering how vital carbon is to life, that it only
comprises 0.3 % of the Earth's crust. Carbon occurs naturally in an uncombined
form as diamond and graphite. Most living organisms from man to the amoeba
(the simplest single cell animal) contain a wide range of organic molecules,
all of which contain carbon. Some minerals contain carbon as carbonates:
examples are chalk and limestone, which are both calcium carbonates, and
dolomite, which is a mixture of magnesium and calcium carbonates. Sedimentary
deposits of plant residues, known as carbonaceous rocks, can include
peat and several different types of coal as well as oil and gas. The composition
depends on the different heat and pressure conditions during the sedimentary
formation.
Carbon is an essential energy source, as oil and coal for the industrial world, and as the simple CARBOHYDRATE, glucose, at the biological level. Plants take atmospheric carbon dioxide and add water to form carbohydrates by a process called photosynthesis. Animals then digest the plants and release carbon dioxide to the atmosphere by respiration. This cycle, linking plant and animal life, is known as the carbon cycle.
Naturally occuring carbon has an ISOTOPE composition (a combination of ATOMS of different weights, designated by the number of sub-atomic particles in their NUCLEI of 12C, 98.89% ; 13C 1.1 % and traces of 14C. Both carbon twelve and thirteen are stable but carbon fourteen is radioactive and decays with a half-life of 570 years. This radioisotope is the basis for radio carbon dating (see ARCHEOLOGICAL TECHNIQUES.).
Carbon does not have a well defined melting point as it tends to sublime, that is to go straight from a solid form to a gas form without passing through a liquid form. The theoretical melting point is very high, above 3500°, and the boiling point is about 4200°. Originally carbon was thought to exist in three allotropic forms: diamond, graphite and carbon black (soot). Allotropy is the variation of physical structure of compounds which have an identical composition. But it is probable that only two of these allotropic forms are different, as the structure of carbon black is very similar to finely powdered graphite.
Diamond
Diamond is a transparent crystalline form
of carbon, and is the hardest substance found in nature. (A new substance
was synthesized in 1957: borazon, which is as hard as diamond and will scratch
it.) Diamond is a poor conductor of heat and electricity; it is 3.5 times
as dense as water. The diamond crystal structure was one of the first to
he determined by X-RAY diffraction. Each carbon atom was found to be surrounded
tetrahedrally (in a four sided figure) by four other carbon atoms, and this
structure spreads throughout the whole CRYSTAL.
So each crystal is a giant molecule. Diamonds that are used in jewellery
can occasionally be coloured by traces of impurities. Many diamonds are imperfect
and these crystals are used industrially. Their extreme hardness gives them
ideal qualities for use in drilling, cutting and grinding. Diamonds are found
mainly in South Africa and Brazil. Because diamond is denser than graphite
it is theoretically possible to convert graphite to diamond. It was only
in the 1950s that the right heat and pressure conditions for conversion were
found. Up to now only small (0.1 carat) high quality industrial diamonds
have been made, none of gem standard. It is also interesting, if the heats
of combustion of diamond and graphite are compared, that graphite is
thermodynamically the more stable allotrope. Diamond can be made to
burn in air if heated to between 600° and 800°C.
 |
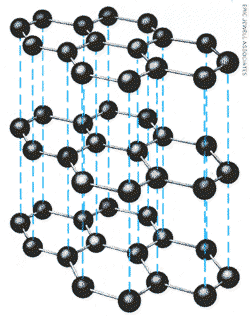 |
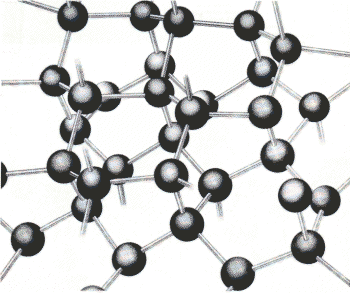
| Pure carbon has two allotropes, or different
solid forms: diamond and graphite. [Note - Recently the allotrope c60 has been found to be a solid form of Carbon] The difference is caused by the way the atoms are arranged. Diamond (left) has atoms that form a stack of tetrahedrons figures with four triangular sides- a shape seen in some milk cartons. This arrangement is reflected in the shape of a complete diamond crystal. Graphite (above) is composed of flat planes of hexagonal rings of atoms; the planes can slide over each other, so graphite is slippery. |
|
Graphite
The other carbon crystal, graphite, is soft, greyish black, slippery
and cold to touch. It is widely distributed in nature and is less dense than
diamond, being 2.2 times as dense as water. Graphite conducts electricity
and heat. It is composed of layers of interconnected planar (flat) hexagonal
rings and as the links between each of these layers are weak, they can slip
over each other with ease. Because of its properties it is used as an
electrode (see ELECTROLYSIS) and for lubrication. These uses are more
important than its generally known use as pencil lead. It also has important
applications as a moderator (for slowing down the speed of neutrons)
in NUCLEAR REACTORS. Many forms of amorphous (without shape) carbon exist,
such as charcoal, soot and lamp black. Examination has shown that these are
microcrystalline forms of graphite. The physical properties of these are
mainly determined by their large surface area. Charcoal is an essential
ingredient of gunpowder, as well as an important purifier because of its
very high surface area, which makes it absorbent. Large quantities of lamp
black are made into printers' ink and carbon paper and added to tyres as
a filler.
 |
| Above: carbon in this form is the hardest substance
found in nature. This rough, octahedral diamond, slightly coloured,
is exactly as it was recovered from the mine. |
Carbon chemistry
The chemistry of carbon is mainly concerned
with its ability to form stable chains of carbon atoms linked together. Study
of these compounds is the basis of organic chemistry. A special point of
carbon chemistry is that the bond between one carbon atom and another is
as strong as the bond between carbon and Oxygen, which makes the chains stable
in air. Carbon combines with sulphur to form carbon disulphide, an extensively
used solvent; with nitrogen to form cyanides; and with chlorine to form a
very stable tetrachloride,which is used as a solvent and cleaning fluid.
Fluorocarbons are used as AEROSOL propellants and as a coating for
non-stick cooking utensils. Certain metals combine with carbon at a very
high temperature, often over 2000°, to produce carbides. Calcium carbide,
CaC2, is sometimes used to prepare acetylene,
C2H2, a gas used in welding. These carbides are often
extremely hard substances,tungsten carbide, WC, for instance, is used as
a cutting edge in many tools. The special properties of steel are the result
of adding carefully controlled quantities of iron carbide to iron. Carbon
forms several oxides: the most well known are carbon dioxide and carbon monoxide.
Carbon monoxide
Carbon monoxide, CO, is a highly poisonous, colourless, odourless gas, which
is made by burning carbon with a limited supply of Oxygen. It is poisonous
because it forms a very strong link with the active iron in the blood
haemoglobin. This stops the haemoglobin from acting as a catalyst
in the respiratory intake of Oxygen. Town gas, the industrially made domestic
gas, contains about 10% carbon monoxide, hence its poisonous nature. But
natural gas consisting mainly of methane, CH4, is harmless from
this point of view. Incomplete combustion of the fuel and air mixture in
cars forms carbon monoxide which can build up to toxic levels in heavy traffic.
Carbon monoxide will combine with certain TRANSITION ELEMENTS to form metal
carbonyls. Nickel forms Ni(CO)4 and iron Fe(CO)5.
Carbon dioxide
The atmosphere contains 0.03%, by volume of carbon dioxide
CO2. It is a colourless gas with a faint odour and tastes slightly
acidic. It may be made by burning carbon with an excess of Oxygen. It is
not poisonous, but as it is heavier than air it will displace it and deaths
have been caused in mines and hollows from asphyxia. Carbon dioxide liquefies
under pressure and freezes at -56.6°. This solid, known as dry ice,
sublimes at -78.5°. Dry ice is widely used when low temperatures are
required. Because carbon dioxide does not support combustion it is frequently
used in fire extinguishers. It is used to prepare fizzy drinks and soda syphons
contain carbon dioxide under pressure which acts as a propellant.
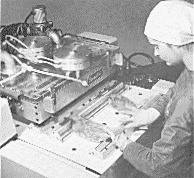
| Top right: a seeding hole produced in a
thick layer of cloud using dry ice, which is solid carbon dioxide. Supercooled
cloud droplets will not freeze spontaneously so dry ice at
-78.5° is dropped onto the clouds to make
them freeze into ice crystals. Bottom right: packaging bacon, cooked meats and cheese in a carbon dioxide atmosphere helps to preserve them. |
 |
||
|
 |
When small amounts of carbon dioxide are dissolved in water the weak and unstable carbonic acid H2CO3 is formed. This gives rise to the bicarbonate and carbonate salts. Sodium bicarbonate, NaHCO3, is baking powder, and can be used as an antacid. Sodium carbonate, Na2CO3, is washing soda.
CARBON COMPOUNDS (see organic chemistry)
Reproduced from HOW IT WORKS p471