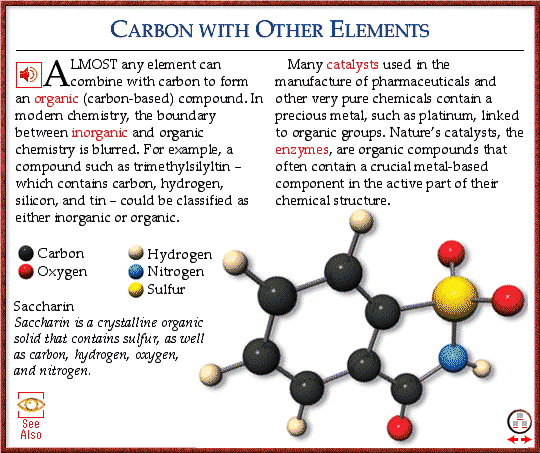
Science |
The Benzene Ring
The structure of benzene was for many years a problem for chemists. Its molecular formula, C_6 H_6 , was established in 1834, but no sufficient model of the structure was provided until many years later.
The Nature of Aromaticity
Examination of the behavior of benzene led Friedrich Kekulé in 1865 to the conclusion that the benzene molecule is symmetrical, and that each carbon atom is directly united to one, and only one, atom of hydrogen. As carbon is assumed to be tetravalent (forming a total of four bonds with neighboring atoms in a molecule),any graphic representation of the molecule should show four bonds between the carbon atom and the atoms directly bonded to it.
This had proved to be a problem for many years, with various linear structures being proposed. It was not until Kekulé produced his resonance structures that a useful model became available.
Kekulé’s Resonance Model
Kekulé initially proposed a six-membered ring structure for benzene in which the carbon-to-carbon bonds were alternately single and double bonds. However, this formula, like others produced around that time, was not wholly satisfactory. He then attempted to find an explanation for the fact that when another atom was substituted for one of the hydrogen atoms, a single compound with a unique structure resulted, but when two atoms were substituted, three isomers were formed. To explain this, he suggested, in 1872, that the double bonds were shared by all the carbon atoms, so that each carbon-to-carbon bond is effectively a single bond half the time and a double bond for half the time:
![]()
This phenomenon is known as resonance, and according to the model the molecule oscillates, or resonates, between the two structures. This remained the most satisfactory explanation of the bonding in benzene well into the twentieth century.
Delocalization of Electrons
Modern theories of bonding since the 1940s involving quantum theory have thrown new light on the whole problem of aromaticity. It is now known that each of the bonds in the benzene molecule has some double-bond character as a result of (pi) bonding.Each carbon atom has four valence electrons with which to form bonds. As in the double bonds of alkene molecules, carbon atoms in benzene are linked by a (sigma) bond, formed by the linear overlap of atomic orbitals, and by a (pi) bond, resulting from the lateral overlap of atomic p orbitals. On each carbon atom in the benzene molecule, these p orbitals overlap with those of both neighboring carbon atoms to form one large molecular orbital, which is often described as an “electron cloud.” Because there are two regions of overlap in a (pi) bond, the electron cloud extends above and below the plane of the ring structure.
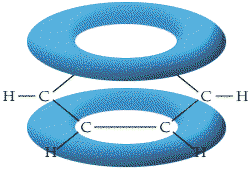
The (pi) Electron Cloud of Benzene
In a conventional double bond, each carbon atom contributes two electrons – one toward the formation of a (sigma) bond, and one to a (pi) bond. In the benzene molecule, each carbon atom is additionally bonded to a hydrogen atom, and since the formation of this bond requires the carbon atom to donate an electron, it has only one electron left (one electron from each atom having already been used in the formation of the C(single bond)C (sigma) bond). These p-orbital electrons (one electron from each of the six carbon atoms) are uniformly distributed, or delocalized, around the ring. Because of the difficulty of drawing a single structure indicating the position of electrons in aromatic rings in benzene and in other compounds, they are often represented in the following manner:
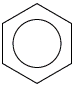
The cyclic, unsaturated structure found in aromatic compounds gives them a chemical stability much greater than that of the corresponding open-chain polyenes. Because of the stability of the ring, aromatic compounds tend to undergo substitution reactions rather than addition reactions (which would disrupt the ring structure by withdrawing electrons to form additional bonds).
Science2 rc Copyright 1994-95, 1997-98 Dorling Kindersley Copyright
1994-95, 1997-98 Dorling Kindersley