

Quantum physics describes the world of the very small. Classical Newtonian
physics describes larger scales. But in the border country between the two,
rigorous mathematical descriptions are difficult to find, and
chaos rears
its head.
MICHAEL BERRY
Before 1900, the foundation of physics was Newtonian mechanics.
The main principle was that forces deflect objects according to laws that
we can describe using simple mathematics. It was natural to think that because
the laws are simple the motion of objects must be simple too. The spectacular
success of classical mechanics in explaining regularities in the motion of
the Moon and planets encouraged this view. It also
inspired the invention of mechanisms imitating those regularities, such as
clockwork.
This view is mistaken. Simple deterministic laws can generate very complicated
and even random motion, because some systems are
so unstable that the course of their trajectories depends sensitively on
how they are started off. Even the motion of a billiard ball the archetypal
Newtonian system can become complicated in certain simple systems. We can
idealize the billiard ball as a point reflected elastically, in other words,
without any loss of energy, from the boundary of the region in which it moves.
Figure 15.1 shows what happens to the ball when we confine it in regions
with different shapes. If the enclosure is a rectangle or a circle, the ball
bounces round in a regular pattern. But if the boundary is shaped like a
stadium or a bulgy Africa', then the ball bounces around chaotically, following
no regular pattern at all.
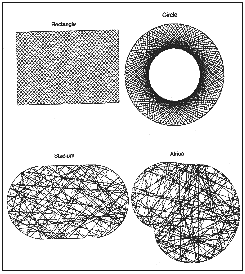 |
| Figure 15.1 The trajectory of a billiard ball depends on the shape of the boundary that confines it. In the rectangle and circle, the orbits are regular; in the stadium and Africa', they are chaotic. |
Nowadays, the 'chaology' of classical mechanics is an intensively
active area of research - chaology is a revival of a term used by theologians
two centuries ago to mean the study of what existed before
the Creation. It has applications ranging from the irregular tumbling
of Saturn's satellite Hyperion to the intricate
orbits of food particles in a liquidizer.
Since the 1920s, we have known that Newtonian mechanics, chaotic or not,
is but an approximation to deeper truths about physics described by
quantum mechanics. When dealing with objects and
processes on atomic and smaller scales, it is quantum, not classical, theory
that agrees with experiment. In its most familiar form, quantum mechanics
is a wave theory. One consequence of this is that
the energy of an isolated atomic system cannot take any value, as in classical
physics. It is restricted to a set of possible energy levels. A common analogy
is with a guitar string, on which waves have a
discrete set of frequencies, or harmonics, that
depend on the string's length, tension and density. The lowest energy corresponds
to the ground state, in which the system usually exists. Higher energies
correspond to excited states. Shining light of the appropriate frequency
onto an atom will drive it into an excited state.
Quantum physics has its own randomness, to be sharply distinguished from
any irregularity that Newtonian trajectories might possess. We cannot, for
example, predict when a radioactive nucleus will decay, or where the next
photon in a laser beam will strike a screen. But from the equations of quantum
mechanics we can calculate with great accuracy the probabilities of these
events from the intensities of the quantum waves. So quantum randomness lies
not in the waves but in the processes the waves describe.
In the everyday world we can see, the direct effects of quantum mechanics
are unobservably small because the wavelengths of the quantum waves are so
small. Even for a bacterium, only a thousandth of a millimetre across, creeping
at one millimetre an hour, the wavelength is a million times smaller than
the bacterium itself - and 100 times smaller than an atom. On scales larger
than an atom, we know that Newtonian mechanics works well, so that quantum
mechanics must give the same predictions, in spite of its very different
conceptual basis.
Niels Bohr, one of the pioneers of quantum physics, saw
this relationship between Newtonian and quantum mechanics as a deep truth,
which he called 'the correspondence principle'. Quantum mechanics must agree
with Newtonian mechanics when applied to large or heavy systems - that is
in the 'classical limit' where we can neglect wave effects. We are familiar
with the principle applied to optics. Light is a wave, but, in explaining
how cameras and telescopes work, it is useful to think in terms of well-defined
rays, very similar to the trajectories of the particles, or 'corpuscles',
Newton envisaged as the constituents of light.
We know that Newtonian physics can give rise to chaotic
behaviour. According to the correspondence principle,
quantum physics is identical to Newtonian physics
in the classical limit. So how does the quantum system reflect this fact?
What features of the way it evolves, and the way its energy levels are
distributed, betray the irregularity of the Newtonian trajectories? Can quantum
systems become chaotic as they approach the classical limit? These are questions
of quantum chaology, an emerging science that is leading to the discovery
of unfamiliar regimes of behaviour in microscopic systems.
The first surprise came 10 years ago, in a theoretical study by the
Italian-Soviet-American collaboration of Giuho Casati at Milan, Boris Chirikov
and Felix Izraelev at Novosibirsk, and Joseph Ford at Atlanta. They investigated
how electrons in highly excited atoms - atoms with electrons in states of
extremely high energy - absorb energy from radiation shining on them. To
avoid tedious computations, necessary for real atoms containing many electrons,
they thought of a simple idealized model of a circulating electron as a bead
on a circular wire, endlessly pursuing its orbit (see Figure 15.2). The waves
of radiation shining on the electron produce an oscillatory force. The
researchers represented this as a sequence of impulses that provided a series
of kicks to the 'bead'. The strength of the impulses depends on the position
of the bead on the circle.
The orbits of this 'kicked rotator', when considered as a Newtonian system,
can be regular or chaotic, depending on the strength of the impulses: stronger
kicks give more chaos.
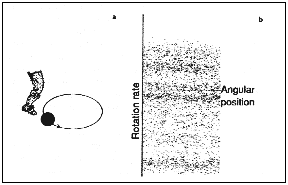 |
| Figure 15.2a The kicked rotator, an idealized model of an excited electron. Figure 15.2b The chaotic 'dust' generated by plotting angular position versus the rate of rotation after 5000 kicks. |
Classical regularity is a steady state in which the rotator,
on the average, gives back as much energy as it absorbs. We also find this
behaviour when we carry out the quantum version of the 'experiment', by making
the bead so light that we have to take into account its wavelength. Classical
chaos, on the other hand, corresponds to erratic diffusion, with the rotator
continuing to absorb energy at a rate which, on average, is constant.
In the chaotic case, however, the corresponding quantum rotator behaves
differently (see Figure 15.3). For a while, the growth of the rotator's energy
follows the classical straight line, but eventually, at a certain 'break-time',
the energy begins to grow much more slowly, and may even decrease. This was
a surprise. Quantum mechanics has suppressed the classical chaos. At first
sight, it looks as though we have a conflict with the correspondence principle.
But when we adjust the quantum model to make it more classical, for example
by making the particle heavier, the break-time, which signals the onset of
nonclassical effects, gets later and later. The theorists discovered this
suppression of chaos by using a computer to solve the quantum equations
numerically. After a decade of study, it is becoming clear that the suppression
is a delicate and subtle wave-interference effect, but physicists have not
yet worked this out in full detail.
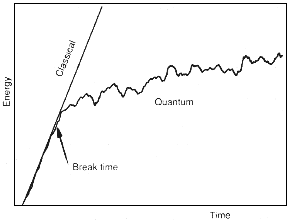 |
| Figure 15.3 After the 'break-time',the quantum rotator absorbs energy more slowly than the chaotic classical rotator. |
A real atom differs from a model rotator in that an electron
can become so excited as to leave the nucleus altogether -thus ionizing the
atom. The probability that a given period of exposure to radiation of a certain
frequency will result in ionization depends on the intensity of the radiation
and how excited the atom is to start with. These conditions determine whether
the classical electron trajectories are chaotic or regular, and whether the
probabilities of ionization calculated using the classical approximation
are the same as those calculated by the more accurate laws of quantum mechanics.
Physicists have carried out experiments on hydrogen atoms illuminated with
microwaves in classically chaotic regimes for which classical and quantum
predictions agree - that is before the break-time. They were surprised to
find systems as small as atoms behaving classically. Remember the absorption
and emission of photons by atoms is a highly non-classical process. The pattern
of emission and absorption lines in a spectrum was one of the observations
that led to the discovery of quantum mechanics.
The difference between the new spectroscopy and the old is that the new
experiments employ intense radiation, and the atoms are in highly excited
states to start with, so they absorb and emit large numbers of photons, rather
than one or two. Theory predicts that, as with the rotator, quantum mechanics
will eventually suppress classical chaos. Experiments to test this important
effect would require that we measure the ionization after much longer periods
of illumination. This is technically difficult and, so far, no one has managed
to do it.
Now we turn to the quantum chaology of systems that are either isolated or
else are influenced by external forces that do not vary - in contrast to
the oscillatory force of radiation just considered. The energy levels of
such systems describe their quantum states. It turns out that the distribution
of highly excited quantized energy levels - the pattern of notes of the harmonics
on the musical analogy - depends in a fundamental way on whether the trajectories
of the corresponding classical system are regular or chaotic.
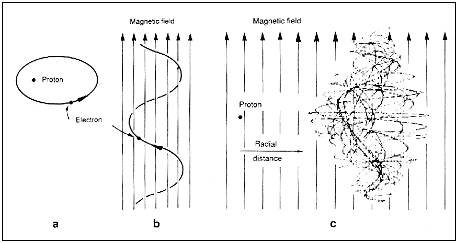 |
| Figure 15.4 Electron orbits: a, regular motion in the field of a proton alone; b, regular motion in a magnetic field alone; C, chaotic motion in a combined field. |
A system encompassing both extremes is the single electron of a hydrogen
atom in a very strong magnetic field - for example, the magnetic field in
a white dwarf star, which can be a billion times greater than the Earth's
field. At low energies, the nucleus of the hydrogen atom, the proton, binds
its electron tightly. The electrostatic force between the proton and the
electron completely dominates the magnetic force. The classical orbits are
ellipses (see Figure 15.4) like the paths of planets round the Sun, and there
is no chaos. At very high energies, the electron is far from the nucleus
and now it is the magnetic force that dominates. The orbits are helices
spiralling round the lines of the magnetic field, and again there is no chaos.
At intermediate energies, however, the two forces are comparable but exert
contrary influences. The classical electron resolves the contradiction by
moving chaotically.
For the electrons behaving in a quantum fashion, we have to compare the
distributions of large numbers of excited energy levels in the regular and
chaotic regimes. One way to do this is by computing the statistics of the
levels. One convenient statistic is the spacings between neighbouring levels,
calculated at low and intermediate energies in the atom's spectrum of energy
levels. If the levels are regularly arranged, like the rungs of a ladder,
the distribution of spacings will cluster about the average spacing, producing
a curve like that in Figure 15.5a. You would get a similar distribution by
plotting the heights of a group of people. In this case there are few small
spacings - it is as though the levels repel each other. If, on the other
hand, the levels are randomly distributed - that is, uncorrelated, like the
arrival times of raindrops in a shower - the distribution of spacings will
be broad, with a preponderance of small spacings. The surprise this time
was the discovery that the levels are more regularly arranged when the classical
orbits are chaotic (see Figure 15.5a), and randomly distributed when the
orbits are regular (see Figure 15.5b). Experiments on magnetized hydrogen
confirm even the fine details of the theoretically calculated spectrum.
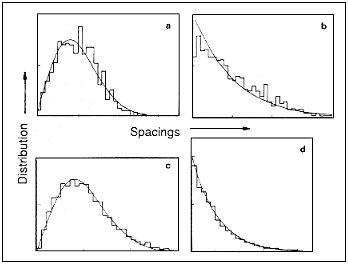 |
| Figure 15.5 The distribution of spacings between neighbouring energy levels: a, hydrogen in a magnetic field with chaotic classical orbits showing repulsion of levels; b, hydrogen in a magnetic field with regular classical orbits showing clustering of levels; C, the energy levels for quantum billiards in a stadium; d, the energy levels for quantum billiards in a rectangle. |
This behaviour is not just a peculiarity of the magnetized hydrogen
atom. On the contrary, the spacings of the quantum energy levels always depend
only on whether the classical orbits are chaotic or regular and not on any
other details of the system. To illustrate this, Figure 15.5 also shows the
distributions of the spacings of energy levels for the quantized versions
of two of the billiard ball games in Figure 15.1. The stadium game is classically
chaotic but has regular spacings of its quantum levels (see Figure 15.5c),
while the rectangle game is classically regular but has random spacings of
its quantum levels corresponding to chaotic motion. These tend to repel one
another. Quantum billiards might appear to be an exotic creation of theorists,
far removed from reality, but exactly the same mathematics describes the
frequencies of a vibrating membrane shaped like the billiard table. In the
three-dimensional version, it also describes the acoustics of a concert
hall.
The repulsion of levels in Figures 15.5b and 15.5d is not the most general
quantum signature of classical chaos, because all systems so far discussed
have a special feature, namely symmetry. The atom in a magnetic field has
the symmetry of a cylinder, and the movement of the billiards is symmetrical
with respect to time, in the sense that, if at any instant the velocity of
the moving billiard ball reverses, it will retrace its previous path. When
there is no symmetry of any kind and the classical orbits are chaotic a
combination of circumstances still out of range of experiment -theory predicts
that repulsion between the levels remains but it is of the slightly stronger
kind shown in Figure 1 5.6a, in which the slope of the curve vanishes at
zero spacing - the curve flattens out. This particular calculation was for
the energies of a charged quantum particle moving in the 'Africa' billiard
table of Figure 15.1 with a magnetic field acting at right angles to the
plane, but it represents quantum chaology in the most general case.
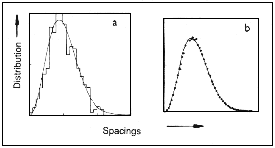 |
| Figure 15.6 The distribution of spacings where there is no symmetry: a, several hundred energy levels of the 'Africa' game of quantum billiards in a magnetic field; b,100 000 zeros of Reimann's zeta function. |
At this point, quantum chaology makes
unexpected contact with one of the long-standing problems of pure mathematics,
namely the Reimann hypothesis of number theory.
In 1859, Georg Bernhard Reimann - a German
mathematician who also developed the study of geometry to include that with
more than three dimensions - was studying the distribution of prime numbers.
He devised a quantity, which he called the zeta function, whose value depends
on position in a plane of complex numbers.
Complex numbers (denoted by s)
have a 'real' part and an
'imaginary' part involving the
square root of minus 1. The x axis represents real numbers and the
y axis imaginary numbers. Reimann's function was the extension to
the whole s plane of
His famous hypothesis was that the points at which the zeta
function vanishes - its zeros - lie on the straight line with x =1/2. (If
the hypothesis were true, certain theorems about prime numbers would follow.)
Numerical studies have shown that the first 1 500000000 zeros lie on Reimann's
line, but nobody has been able to prove that they all do (see Figure 15.7).
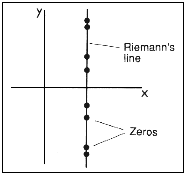 |
| Figure 15.7 The plane is inhabited by Reimann's zeta function.His hypothesis is that the function's zeros lie on the line shown. |
The connection with quantum chaology comes in calculations
by Andrew Odlyzko (AT & T Bell Laboratories, New Jersey) of the distribution
of the spacings between neighbouring zeros; this takes 20 hours of Cray
supercomputer time and results in the graph in Figure 15.6. It is not just
the evident similarity between Figures 1 5.6a and 1 5.6b, but also a variety
of other evidence, which suggests that underlying Reimann's zeta function
is some unknown classical, mechanical system whose trajectories are chaotic
and without symmetry, with the property that, when quantized, its allowed
energies are the Reimann zeros. These connections between the seemingly disparate
worlds of quantum mechanics and number theory are tantalizing.
The phenomena of quantum chaology lie in the largely unexplored border country
between quantum and classical mechanics; they are part of semiclassical
mechanics. This is an area where rigorous mathematical development, as employed
elsewhere in mechanics, is difficult. Most discoveries have been made by
computer experiments with the quantum equations, guided by intuition and
analogy. As the subject matures we can expect, on the one hand, more experiments
on real physical systems, and, on the other, the precise formulation and
proof of mathematical theorems.
| Chaos | Quantum | Logic | Cosmos | Conscious | Belief | Elect. | Art | Chem. | Maths |
New Scientist 28 Oct 1989 File Info: Created 16/7/2013 Updated 10/9/2017 Page Address: http://leebor2.100webspace.net/Zymic/edgechaos.html