
 |
How it works : Electricity |
ELECTRICITY
Electricity, as its name suggests, is intimately bound up with the properties and consequent behaviour of the ELECTRON. The charge on the electron and its relatively high mobility, the ease with which it can be caused to move, are its most important properties from the point of view of electricity. It is the motion of electrons that lies at the root of all electrical phenomena, as, for example, in a current in an electric CIRCUIT.
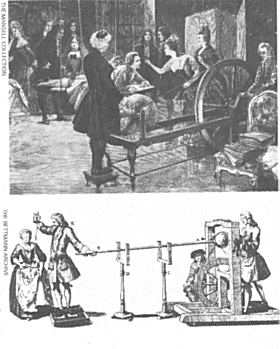
Below: demonstrations of electrical phenomena were popular in 18th century France. Here Abbi Nollet (1700-1770), an associate of du Fay, is demonstrating the effects of static electricity. Bottom: von Guericke discovered, in the 1660s, that an electrical charge could be produced by rubbing a rotating globe of sulphur. This machine, devised by G M Bose, used a globe of glass. |
 |
Electrons are found in all ATOMS, surrounding the compact core, or nucleus, of protons and neutrons in orbital layers or 'shells'. The ELECTROSTATIC attraction between the protons and electrons holds the atom together. The electrons in the outermost shell, which are often termed the VALENCY electrons (those which take part in chemical BOND formation), are only weakly bound to the nucleus in many atoms. This is especially the case with the valency electrons of the atoms of the metallic elements, such as copper, silver, and sodium. The weakness of the attraction, combined with the fact that the mass of both the proton and the neutron is each nearly two thousand times that of the electron, means that the valency electrons can be relatively easily moved. Since electrons are present in all atoms, electricity, broadly defined, is found in many forms throughout nature. Photo-electricity, the movement of electrons from their atomic levels by the action of light, and the whole field of electro-chemistry (see ELECTROLYSIS) are examples of the extent to which the effects of the electron's properties are encountered.
Current
When the atoms or molecules of an element or compound come together,
they arrange themselves in a regular array termed the
CRYSTAL lattice. The type of crystal lattice and
the mobility that it allows the valency electrons, and the nature of the
particular atom itself, largely determines the electrical properties of the
element or compound. RESISTANCE, for example, is a measure of the
amount of energy expended by the electrons in moving against the opposition
of the atoms in the lattice. This expended energy appears mostly in the familiar
form of heat. CAPACITORS exploit the low electron mobility of insulators
to build up a surplus of electrons on one of their plates and a deficiency
on the other. This imbalance of charge can later be 'discharged' in a short
pulse of current.
The lattices of metallic elements allow the valency electrons a high degree of freedom and present little opposition to their motion. As a result, they are excellent conductors of electrical current. Insulators, on the other hand, are characterized by the low mobility their atoms and lattices allow the valency electrons (see CONDUCTION, electric).
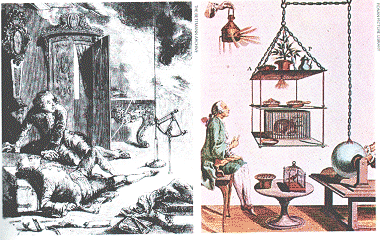
| Below left: lack of knowledge made many early electrical experiments dangerous and unpredictable. This engraving shows George Richmann after be was struck by lightning during an experiment. Below right: another of Abbi Nollet's experiments, investigating the effects of high voltage electricity on plants and animals. The electricity was conducted along the chain supporting the specimens. |
 |
The crucial importance of the type of crystal lattice is demonstrated by the case of the two distinct crystalline forms, or allotropes, of pure carbon. In the graphite form, carbon is an excellent conductor, but in the diamond form it is a poor conductor. The atoms making up the two lattices are exactly the same, but the electron mobilities allowed by the lattice types are completely different.[Note: As of today THREE allotropes are known -the third being c60]
Individual electrons move along solid conductors in a random 'shunting' manner; it takes a flow of over six million million million electrons per second to make a current of one ampere, named after the French physicist A M AMPÈRE. While the effects of a current (such as ELECTROMAGNETIC RADIATION) are transmitted with the speed of light, each electron is only drifting in the direction of the current at an effective rate of around one inch per second (2.54 cm per sec). When ionic compounds, such as common salt (NaCl), are dissolved in water, the water molecules cause the sodium and the chlorine atoms to split up, or 'dissociate', into free IONS. The negative chloride ion, which carries the single valency electron in the outer shell of the sodium atom, and the correspondingly positive sodium ion are then able to carry a current through the solution. Instead of the mobility of the electron, it is the mobility of the ions through the solvent (in this case water) that determines the resistance of the solution.
Voltage
Although the electrostatic attraction between the proton and the electron
is one of the fundamental forces of the Universe, the systematic motion of
electrons that constitutes a current is not directly caused by it. Current
flows between points of 'potential difference', this difference being expressed
in volts. The voltage between two points is a measure of the energy
an electron gains in moving them: the electrons move because it is preferable
for them to do so, and the energy arises as a result of the 'energy levels'
the electron can fill in the atom. The complete theory of electricity, which
clearly hinges around the atomic and QUANTUM theories
of matter, has only evolved during this century. But the scientific observation
of electrical phenomena, and even their exploitation in practical applications
such as the BATTERY, long preceded this full understanding. This is another
example of a common occurrence in science: the laws of nature have often
been put to good use even though the principles underlying them were either
unknown or misunderstood.
Triboelectrification
The first record of the study of electricity can be found as early as
the sixth century BC. A Greek, Thales of Miletus (624-548 BC), observed that
when certain substances were rubbed they were able, for a time, to attract
light objects. Most people are familiar with this phenomenon in one form
or another: the effect a dry nylon comb has on hair, making it stand on end,
is one example. Also when the plastic body of a fountain pen is rubbed with
a silk, cotton, or woollen cloth, it can pick up small pieces of paper. This
is called triboelectrification, and it is one of the most fundamental
results of the electrical properties of matter.
| Below: a sheet of 'Perspex' was charged up by scanning a high energy beam of electrons across it, and when it was earthed (grounded) at one edge this spectacular discharge pattern occurred. |
 |
The triboelectrification of an object is due to the frictional force applied in the rubbing process. Certain types of atoms can be ionized like this, being either partially stripped of their valency electrons or given some of the valency electrons of another atom. The cloth that is used to induce this ionization,as could be expected, is also ionized-with the opposite charge. It, too, has the ability to attract light objects for a while. This ionization, however, is not at all extensive, and the normal balance of positive and negative charge is soon restored when the object is 'earthed' [grounded]. This can be done by the action of the moisture in the surrounding air or by being simply touched.
Magnetism and polarity
The word 'electron' is actually derived from the Greek word for
the natural resin amber, elektron. Amber was one of the first substances
to be triboelectrified. The Greeks connected this phenomenon with magnetism,
which they had observed between pieces of magnetite ore. Since both this
force of electrostatic attraction and the force of magnetism act 'at a
distance'-that is, do not require any physical contact to pull objects
together-they seemed to fall into one class. Thus, from the outset, the
scientific studies of electricity and magnetism were linked, although for
a spurious reason. The first genuine connections between the two were not
made until 1820, when Oersted chanced to see that a COMPASS needle was deflected
by the presence of a current- carrying wire. Strangely enough, one school
of Greek thought did develop a simple version of the atomic theory, (see
EPICURUS) but the link between electricity and the atom did not come until
the turn of this century.
In 1551 Jerome Cardan studied the similarities and differences between magnetic and electrostatic attraction. He proposed that electricity or, rather, the triboelectric aspect of it that he was concerned with, was a type of fluid. All things considered, it is not such a bad model. What was more important, it meant that this mysterious force was now treated as something quite material and not at all magical. Fluid theories of electricity became popular in the 18th and early 19th centuries.
In 1600 William Gilbert, an early investigator into electrical and magnetic phenomena, conducted extensive experiments into triboelectrification. He classified substances into good or poor electrifiers, depending on how easy it was to induce in them this ability to attract light objects. His classifications can be seen to correspond to the modem ones of 'insulators' and 'conductors' respectively.
The fluid theories of electricity were subsequently developed further by the French scientist du Fay. In 1733 he proposed that there was only one fluid responsible. In 1747 Benjamin FRANKLIN, in his celebrated experiments with kites flown during thunderstorms and an early type of capacitor called a Leyden jar, proposed that there were two types of electrical fluid. One was responsible for 'positive', the other for 'negative' electrification.
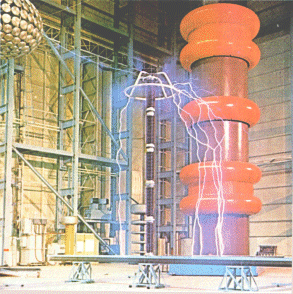
| Below: the 'flashover' discharge created during the testing of a high voltage insulator, of the type used in power stations, transforming stations and switching compounds. |
 |
This distinction between positive and negative came about because some electrified substances attracted each other, whereas others were repelled by them. This again is another obvious parallel with magnetism: a north pole of a magnet repels another north pole, just as a south pole repels another south pole, but a north pole and a south pole attract each other. The fluid theories, however, were subsequently discarded with the advances in scientific knowledge in the 19th century but the 'positive' and 'negative' terminology was retained along with the 'like repels, unlike attracts' rule.
Coulomb's law
The quantitative development of electrical theory began in the second
half of the 18th century The independent discovery, by Priestley in 1767
and COULOMB in 1785, of the so called Coulomb law, and its exact expression
in a mathematical formula, started it. The Coulomb law is to electricity
what NEWTON'S LAW of gravitation is to physics,
and in fact it is remarkably similar in its general form.
Coulomb's law relates the electrostatic force of attraction or repulsion between two groups of charge to the amount of charge, the distance separating them, and the medium in which they are situated. The force was found to vary with the reciprocal of the square of the distance between the charges. This means that doubling the separation will reduce the force to one quarter of its original magnitude. The electrostatic force is thus a 'short-range' force, as it dies away in intensity very rapidly with distance. This formula has stood essentially unchanged to this day, a tribute to the experimental precision of both scientists.
From then on the understanding of electricity broadened rapidly, due mainly to the invention of the first reliable source of continuous current. This was called the 'voltaic pile', and it is the forerunner of the modern battery. In fact, it relied on the same current-generating process as the modern battery-the controlled electrolytic action of a weakly acidic solution on metals. Its invention in 1800 by the Italian physicist Alessandro VOLTA, after whom the 'volt' is named, was a turning point in the study of electricity. Before the voltaic pile, the only way of 'storing' charge was a bank of electroscopes (devices for storing electrostatic charges), the current from which was small and usually too short-lived to allow its effects to be studied in any detail. The electroscopes were each laboriously 'charged' by repeatedly transferring the charge from a triboelectrified substance such as amber or glass on to the electroscope.
| Below: lightning is the most spectacular natural form of electricity, produced by the discharge of electricity between highly charged clouds and the earth. This picture was taken at Santa Fé, New Mexico. |
 |
Electromagnetism
The chance discovery of the relationship between magnetism and an electrical
current by Oersted in 1820 was the next breakthrough. The brilliant British
physicist Michael FARADAY soon after discovered that the relationship was
two way. Not only does the motion of charge that takes place in a current
produce a magnetic field in its vicinity but, conversely, the 'motion' of
a magnetic field produces a current. In 1831 he showed that the continuous
variation of the magnetic field, or 'flux', passing through a conducting
wire loop causes a current to be circulated in the loop. The mutual dependence
of electricity and magnetism is the principle behind what is now termed
INDUCTANCE.
In 1851 Faraday realized that it is not just the actual motion of the electrons that lies at the centre of electricity. Rather, it is the electric and magnetic 'force fields' they set up as they move along. In 1865 James Clerk MAXWELL published his theory of electromagnetism, which related the two in an exact, mathematical fashion.
The rapid development of the atomic theory, with its discovery that all matter is ultimately composed of, among other particles, electrons, meant that electricity was afforded a central role in physics. In 1887 Hertz showed that light was merely a travelling electromagnetic wave, and thus optics was seen to be basically a branch of electrical theory. When J J Thomson provided the first positive demonstration of the existence of the electron in 1897, the ancient enigma of electricity was finally explained.
During this century, both an understanding of the fundamental, atomic-level principles of electricity and the development of its applications have increased dramatically. For example, the ability to generate large quantities of electrical power, such as through the 'harnessing' of running water for hydroelectric power, and the solution of the related problems of its wide- scale distribution, has brought vast changes in the standard of living. The invention of thermionic vacuum devices such as VALVES [vacuum tubes], and the more recent work on SEMICONDUCTORS, has opened up the science of ELECTRONICS.
ELECTRICITY SUPPLY (see power supply)
Reproduced from HOW IT WORKS p846