Buckyballs open a whole new game
The merest specks of soot are sweeping chemists of their feet
 |
| Why God had a ball making the universe |
| God, it seems, loves the beautiful game - when he fashioned
the universe he made it the shape of a football. The cosmos is not infinite
- but a finite 'soccer ball' of curved pentagons
joined together, scientists claimed yesterday. In an attempt to kick
into touch all other theories about the shape of the universe, NASA experts
said it was 'positively curved' . They described it as like a
hall of mirrors, endlessly
reflecting itself . In this closed dodecahedron
- a 12-faced object - a spacecraft exiting through one pentagon would
re-enter the same region through the opposite face and would meet the same
galaxies over and over again. If confirmed, the results would mean the universe
is much smaller than believed and about 70 billion light years across. The
findings are based on data collected by a NASA spacecraft, the Wilkinson
Microwave Anistropy Probe, mapping the radiation left over from the
Big Bang. Current cosmos
theories include one which proposes the Earth's 'local' universe is one
of many expanding bubbles. Other observations suggest
that it is 'flat' - meaning parallel lines stay the same distance apart and
never meet. Another view is that it is spherical but in three dimensions
- so if you could look through a very powerful telescope you would see the
back of your head. The new theory challenges all those ideas, says leading
US mathematician Dr Jeffrey Weeks. 'Since antiquity humans have wondered
whether out universe is finite or infinite,' he told journal Nature. 'Now,
after more than two millennia of speculation, observational data might finally
settle this ancient question. [The Metro 9Oct 2003] |
When a candle burns,it is the beauty
of the bright flame that attracts our attention. But ask a chemist where
the real beauty lies and the answer will come as a surprise: it is in
the candle's soot.
For lurking in the black deposits are tiny amounts of buckminsterfullerene,a
ball like arrangement of carbon atoms discovered in 1985 and now considered
by chemists to be The Most Beautiful Molecule.
It is 10 years since their discovery but
"buckyballs"
- which are named after the innovative American architect Buckminster
Fuller,whose domed structures bear an uncanny likeness to the molecules -
are still one of the hottest topics in chemistry.
There are many possible applications for the
buckyballs,ranging from rocket fuel to anti-Aids
medicine,and new discoveries about them are emerging in research literature
at the rate of around one a day.
Yet the story of their discovery is a classic case of "useless" science
producing something quite unexpected and of huge commercial potential.This
week sees the publication of two books describing that story.
According to Dr Jim Baggot,author of one of them - Perfect
Symmetry
:the accidental discovery of buckminsterfullerene (Oxford University
Press) - the first inklings of the existence of buckyballs appeared in,of
all places,a humorous article in the New
Scientist magazine in 1966.
Written by David Jones,himself a scientist,the article centred on a curious
"desert" of materials with densities somewhere between those of gases - which
are typically a thousand times less dense than water - liquids and solids.
Mr Jones suggested that molecules of a material like carbon might,if curled
up like a ball,bridge this huge gap.
His suggestion proved astonishingly accurate.In 1985,Dr Harry Kroto of Sussex
University was working with colleagues at Rice University in Houston
investigating the apparently esoteric problem of forming large carbon
molecules in deep space.
They were trying to explain observations suggesting that clouds
of such molecules were lurking in the cosmos,mopping up light from stars.
Dr Kroto and his co-workers tried to make these large molecules in
the laboratory by blasting graphite using laser beams.
They discovered that,for some reason,they were producing a preponderance
of one type of carbon molecule,containing 60 carbon atoms.
Why so huge a molecule should be found in such numbers was a complete
mystery: its chemically reactive "edges" should have led it to almost immediate
annihilation.
But then Dr Kroto and his colleagues had a brainwave: perhaps the molecule
was folding up inot a ball,thus connecting up all its reactive edges.
After much playing with cardboard models,the team finally succeeded
in getting the right shape.
Carbon- 60 is indeed a ball of carbon atoms,its surface made up of hexagons
and pentagons - just like a football.

According to Hugh Aldersey-Williams,the author of the second
book on the discovery (The Most Beautiful Molecule,Aurum Press),this
led the research team to consider names like ballene,spherene and soccerene
In the end the unwieldy buckminsterfullerene has,however stuck - and deprived
headline writers everywhere of a feast of invention.
Now the search is on for applications.By trapping atoms inside the
"cage" of buckminsterfullerene,scientists hope to create molecular switches
and memories for storing data.
By adding impurities,they have also found that bucky balls become
"superconductors",losing
all their electrical resistance at very low temperatures.
Both may have long-term technological uses.
| WAV 259K |
Researchers at the University of California last year showed that a water-soluble
form of the molecule fits neatly into the crevices of the Aids virus,blocking
its activity.
Among the more bizarre applications is rocket fuel: by accelerating them
electrically and ejecting them out of the back of a spaceship,the relatively
dense and pellet-like buckyballs may be an ideal propellant for very long
spaceflight.
Technologists are now trying to turn such possibilities into reality.If they
succeed,it will be a salutary lesson in the value of "useless" science.
"At the root,this is a story of classic "bootleg" science," points out Mr
Aldersey-Williams."The work was done on the back of other funded projects
and when time would allow.Yet its commercial implications are probably
immense."

Robert
Matthews
Sunday Telegraph
Faux Fullerenes
Like a newly learned word that seems to jump from every book
molecular cages have become ubiquitous since the existence of
buckminsterfullerene's icosahedral carbon cage was confirmed two years ago.
First came larger carbon cages called giant fullerenes; nested cages, known
as Russian dolls; and ultrathin fibers, called
buckytubes. Next were
the metallofullerenes-hybrids that encase metal atoms or incorporate them
in the carbon lattice itself. Now the synthesis of a carbonless envelope
has been announced: a nested cage of tungsten disulfide [see illustration
below].
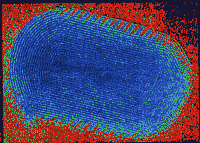 |
| Uncarbonated Fulleroid consists of nested cages of
the semiconductor tungsten disulfide. |
The faux fullerenes first appeared in July 1991 at Israel's
Weizmann Institute of Science where Reshef Tennep Lev Margulis, Menachem
Genut and Gary Hodes were preparing tungsten disulfide for use in
high-performance solar cells. The workers did not immediately grasp the
importance of the nested balls of the semiconductor material. "We saw the
Russian dolls in July 1991 ," Tenne says, "but we did not make the connection
until later, when we looked at the pictures made by Iijima." (Sumio Iijima
of NEC Corporation described nested buckytubes late in 1991.)
As a result of the delay,the Weizmann researchers can state categorically
that the mock buckystructures are stable for at least a year. But easy though
they may be to keep, no one has yet produced them in bulk. Like their carbon
archetypes phony fullerenes form only at high temperatures. In such a regime
a vapour of tungsten disulfide condenses into a two-dimensional sheet, as
do the carbon precursors of fullerenes. Some hexagonal cells then convert
to pentagons, causing the sheet to curve in on itself and close.
What tricks might these motes perform if they could be made
by the gram? "I guess they will show photoconductive and quantum effects,"
Tenne says The smallest cages of tungsten disulfide are believed to have
an electronic band gap well below the 1.6 electron volts of the bulk material.
"As the number of layers rises" Tenne notes "the gap should approach that
value." Materials scientists can therefore hope to control the growth of
the structures so as to "tune" the band gap for their electronic properties.
For example one might tune the Russian dolls for optimal absorption of sunlight,
producing better solar cells. Even more exciting is the prospect of tuning
tungsten disulfide so that it emits visible light. The bulk form of this
material cannot serve this function because it is like silicon, an indirect-gap
semiconductor, in which electrons and positive charges or holes, do not normally
recombine to form light.
Other possibilities also beckon. Tungsten disulfide is used
as a lubricant in some aerospace applications. If it retains this property
in its fulleroid form, it may serve to grease the wheels of
tomorrow's
nanomachines. One might for example deposit tiny greaseballs in a microscopic
bushing or inside a minuscule ball-and-socket joint. Mock buckytubes might
also be intercalated with lithium to form microscopic rechargeable batteries.
The range of properties of fakeyballs looms even larger because other substances
can also condense into sheet-like precursors. Each substance might father
an entire family of shapes and sizes. "Oh, there are so many two-dimensional
materials" Tenne exults. "We are trying molybdenum disulfide. Then we will
go to other compounds."
-Philip E. Ross
[Scientific American Feb93]
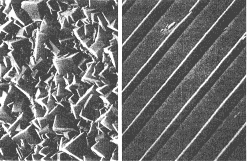
Buckyball film is no rough
diamond
Thin films of buckyballs have been grown by physicists in the
US. The films are smooth and soft (right picture) , quite unlike diamond
films, which have a hard, jagged surface (left).
 |
The C60 films are between 200 and 300 nanometres thick. They
were made by Art Hebard and his colleagues at AT&T's Bell Laboratories
in Murray Hill, New Jersey. According to Hebard, the nanometre-sized buckyballs
sublime easily when heated and deposit themselves on nearby surfaces.
Buckyballs
are neutral overall but because they have regions of positive and negative
charge, they can attract each other.
Hebard says that when a buckyball is in contact with a surface, electrons
move from the surface to the buckyball, forming an ionic bond. On a silicon
surface buckyballs tend to bond where they land. But on gallium arsenide,
a semiconductor which is like silicon but which has a different arrangement
of electrons, the buckyballs are very mobile.
The grooved pattern in the picture was etched using a technique
similar to that used to print circuit patterns on silicon chips. This method
involves placing a material called a photoresist on the silicon surface of
the chip and then placing a mask, which contains the circuit pattern, above
this.Next ultraviolet, light is shone onto the mask. Wherever the light shines
through the mask it strengthens the chemical bonds in the photoresist layer
below. This means that when the surface is rinsed with a solvent, it washes
away only those parts of the photoresist which were unexposed to light.
Hebard found that, just like photo resist, C60 is sensitive to ultraviolet light. By shining a striped pattern of ultraviolet light onto the film, he was able to strengthen the chemical bonds in the exposed areas, making them resistant to solvent. AT&T has not yet revealed details of etching process. Hebard says there is no known use for patterned C60 films.
Elisabeth Geake
[New Scientist
12 June1993]
Non-metallic magnet could be dream computer memory
19:00 17 October 01 Justin Mullins
A transparent, flexible magnetic material made from an exotic form of carbon could turn out to be the dream computer memory. The substance, which was discovered accidentally by a Russian physicist hunting for high-temperature superconductors, is the first non-metallic magnet to work at room temperature.
Tatiana Makarova, working at Umeå University in Sweden, discovered the material while experimenting with buckyballs, football-shaped molecules made up of 60 carbon atoms. By heating and compressing the molecules, she forced them to join together in layers like sheets of bubble wrap, because she thought these might be able to superconduct.
But to her surprise, she found instead that the new material was magnetic even above 200 °C. Until now, the highest temperature at which a non-metallic material was magnetic was 255 °C. This record was held by a different form of buckyballs.
Organic magnets could be important because they are much lighter than their metallic cousins. Also, Makarova's material is flexible and transparent, properties that could make it useful for storing data when a laser is used to record on it. It might also be possible to record data at unprecedented densities.
Mystery magnetism
Exactly why the material is magnetic is not yet clear. Makarova believes that unpaired electrons may play a crucial role, since they can sustain a magnetic field when their spins are aligned. One possibility is that the magnetism stems from buckyballs bonding in triangular groups of three.
"In this configuration, there can be unpaired spins," she says. Her team is currently comparing buckyball layers made in different ways to try to find out.
Robert Blinc, an expert on molecular magnets at the University of Ljubljana in Slovenia, says the work is a giant step forward. He says it is not yet clear whether the magnetic properties are uniform throughout the structure or occur in clumps. "But in any case it is extremely important," he says.
Journal reference : Nature (vol 413, p 716)
19:00 17 October 01
TECHNOLOGY
Warmer Superconducting Buckyballs
Image: courtesy of J. Lauher's Fullerene Structure Library
The marvel of superconductors is their ability to carry electricity without any loss. A superconducting ring, for example, can theoretically sustain a looping current indefinitely. The catch, however, is that most superconducting materials work only when they are supercold: traditional superconductors must be chilled to within a few degrees of absolute zero, and even so-called high-temperature superconductors--ceramics made with copper and oxygen--need to reach the temperature of liquid nitrogen, or negative 196 degrees Celsius, before they start to hum. But new hope for even higher temperature superconductors has emerged in the form of carbon soccer balls called buckminsterfullerenes, or, simply, buckyballs: scientists from Bell Laboratories report in today's issue of Nature that it may be possible to create buckminsterfullerenes that are superconducting at practical temperatures.
In the early 1990s, scientists found how to make buckyballs superconducting
by doping them with metal atoms. The metals, trapped inside the buckyball's
carbon sphere, release free electrons that carry current. But the highest
temperature at which these metal-doped buckyballs became superconducting
was about negative 255 degrees Celsius. Now
Bertram
Batlogg and his colleagues have raised that threshold by another 40
degrees--simply by doping the buckyballs in a different way. Instead of adding
electrons with metal dopants, they removed them by injecting "holes" to molecules
of C60--buckyballs containing 60 carbon atoms (see image). Holes, too, carry
current and act much like electrons, except that they have a positive charge.
When the scientists added about three holes per molecule of C60, the buckyballs
became superconducting at negative 221 degrees Celsius. And they suspect
they can raise that temperature even higher--perhaps well above that of liquid
nitrogen--by using some inert dopant to push the individual buckyballs farther
apart. --Kristin Leutwyler
[Scientific American
31/12/2001]
![]() MORE NEWS NANOTECHNOLOGY
MORE NEWS NANOTECHNOLOGY
Carbon Nanotubes Could Lengthen Battery Life Carbon nanotubes—tiny
tubular structures composed of a single layer of carbon atoms—could
lengthen the life of batteries, according to new research. Findings published
in the current issue of Physical Review Letters suggest that the diminutive
tubes can hold twice as much energy as graphite, the form of carbon currently
used as an electrode in many rechargeable lithium batteries. The reduction
and oxidation reactions that occur at the electrodes of batteries produce
a flow of electrons that generate and store energy. Conventional graphite
electrodes can reversibly store one lithium ion for every six carbon atoms.
To investigate the storage capacity of carbon nanotubes, Otto Zhou and colleagues
at the University of North Carolina, Chapel Hill, first created bundles of
the single-walled straws. They then shortened the tubes and opened their
ends by immersing them in strong acids. Subsequent tests of their energy-holding
potential, conducted using electrochemistry and nuclear magnetic resonance
spectroscopy, revealed an electrical storage capacity approximately double
that of graphite. In explanation, the scientists note that the tubes' open
ends facilitated the diffusion of lithium atoms into their interiors. Indeed,
the tiny straws managed to reversibly store one charged ion for every three
carbon atoms. As with many findings in the nascent field of nanotechnology,
commercial devices based on the work remain a ways off. "We'll have to work
on and overcome other practical issues before we can make real devices,"
Zhou says, "but we are very optimistic." —Sarah Graham
[Scientific American ]
![]() NANOTECHNOLOGY
NANOTECHNOLOGY
Carbon Nanotubes Could Serve as Ultrafast Oscillators
 The
minuscule size of carbon nanotubes—hollow cylinders measuring a few
billionths of a meter wide—can boggle the mind. Now picture the straws
nestled inside one another like Matryoshka dolls, with the inner set of tubes
sliding in and out a billion times a second. In fact, according to a report
published in the January 28th edition of Physical Review Letters, scientists
could conceivably fashion just such a gigahertz oscillator—one that
could aid in the creation of nanomechanical devices.
The
minuscule size of carbon nanotubes—hollow cylinders measuring a few
billionths of a meter wide—can boggle the mind. Now picture the straws
nestled inside one another like Matryoshka dolls, with the inner set of tubes
sliding in and out a billion times a second. In fact, according to a report
published in the January 28th edition of Physical Review Letters, scientists
could conceivably fashion just such a gigahertz oscillator—one that
could aid in the creation of nanomechanical devices.

The curious 60-atom clusters of carbon, may have found a medical
niche. Modified versions of the hollow carbon balls could protect injured
brain tissue by mopping up highly reactive molecules called free radicals,
say researchers in the US. |
Previous work by John Cumings and Alex Zettl of the University of California, Berkeley, had shown that prying open one end of such a multiwalled nanotube transformed it into a telescoping tube: the inner tubes slid in and out of the surrounding straws with very little friction (see image). In the new study, Qing Jiang of the University of California, Riverside and Quanshui Zheng of Tsinghua University in Beijing modeled the sliding action of a tube with two open ends. Their calculations show that if the inner core were pulled out of such a tube, it would not only retract back into the center of the tube, but it would also continue right out the other end. Nearly negligible friction between the tubes would enable a breakneck gigahertz oscillation frequency, the investigators report. And shorter tubes could move at even greater speeds.
Jiang suggests that the tubes may one day make their way into fiberoptic systems as ultrafast optical filters. Before that can happen, however, researchers will have to figure out exactly how to excite the oscillator and how to couple it with the rest of the nanoscopic device. "The actual implementation of this coupling," the authors conclude, "represents another challenge in development." —Sarah Graham [Scientific American 23/1/2002]
Osmium Is Stiffer than Diamond, Scientists Discover
Whether it will compete for the title of a girl's best friend remains to be seen, but the element osmium can already challenge diamond in at least one respect: stiffness. According to a report published in the current issue of Physical Review Letters, osmium can withstand compression better than any known material. The results provide a potentially new lead in the search for superhard materials.
Diamond's ability to resist scratches, dents and chipping--in short, its hardness--makes it an ideal choice for tips in industrial-strength machines. A related quality that is easier to calculate than hardness is an element's resistance to compression, known as its bulk modulus. The properties are interrelated because the stiffest materials also tend to be the hardest ones. But even though osmium is much softer than diamond, initial estimates of its bulk modulus indicated a similar value to that of diamond.
Hyunchae Cynn and colleagues at Lawrence Livermore National Laboratory thus
set out to test the property experimentally. They squeezed osmium powder
under 600,000 atmospheres of pressure and calculated changes in the spacing
between atoms in the sample using x-ray diffraction patterns. The team reports
that osmium's bulk modulus is 462 gigapascals (GPa), as compared with diamond's
443 GPa. "It is intriguing that a light, covalently bonded element such as
diamond and a heavy, metallic element such as osmium, with very different
chemical bonding, would both have large values of the bulk modulus," the
authors note. They conclude that related compounds such as transition metal
carbides, nitrides and oxides could be sources of new superhard materials.
--Sarah Graham [Scientific American
2/4/2002]
16:02 21 May 02
NewScientist.com news service
Researchers at IBM have fabricated a carbon nanotube transistor that mimics the design of modern silicon transistors but performs much better.
Transistors are the "switches" in electronic circuits, controlling the current that represents digital information. Making transistors smaller means more can be packed into circuits, resulting in more powerful computer chips.
But silicon transistors are expected to reach their physical limit of miniaturisation in the next couple of decades. Researchers hope nanotube devices may help keep circuits shrinking towards the atomic scale.
Engineers at IBM's Watson Research Center in New York fabricated a single-walled carbon nanotube field-effect-transistor (CNFET) designed to mirror the architecture of a metal-oxide semiconductor field-effect-transistor (MOSFET). The later is very common in modern silicon circuits.
Shalom Wind, at IBM, says the new nanotube transistor differs from previous designs. He says its gate, which controls the current, is on top of a nanotube and separated from the channel by a layer of silicon dioxide, rather than being shared between a number of transistors. This makes it possible to control each transistor individually.
Current boost
The research team found that the nanotube transistor's gate could control the current better, meaning larger currents could be used. "The more current you can drive, the more flexibility you have in circuit design," Wind told New Scientist. "The implication is that you could have faster transistors."
The transistor's novel design also means the researchers were able to fabricate transistors with different polarity. This is a crucial property of complementary metal oxide-semiconductor (CMOS) circuits, which are also very common.
Cees Dekker, a carbon nanotube researcher at Delft University of Technology in the Netherlands, says this is an important step towards building practical nanotube circuits. "It's interesting to make the comparison with silicon," he says. But he warns that many parameters are important in determining the efficiency of a transistor.
At present, the nanotube transistors are not smaller than the silicon ones, but the IBM researchers claim their results are a proof of principle and that the nanotube transistors will outperform them even when shrunk.
Journal reference: Applied Physics Letters (vol 80, p 3817)
Will Knight
![]() DIAMOND FILM COULD FORM
BASIS OF HIGH-POWER ELECTRONICS They've long been a girl's best friends,
but now diamonds might just win over electrical engineers, too. According
to a recent report, scientists have succeeded in fabricating films of synthetic
diamond that have properties similar to those of silicon. The results could
lead to new carbon-based electronics.
http://sciam.rsc03.net/servlet/cc?lJpDVUWEmLtisHklLkpLlFHhsDJhtE0EV
DIAMOND FILM COULD FORM
BASIS OF HIGH-POWER ELECTRONICS They've long been a girl's best friends,
but now diamonds might just win over electrical engineers, too. According
to a recent report, scientists have succeeded in fabricating films of synthetic
diamond that have properties similar to those of silicon. The results could
lead to new carbon-based electronics.
http://sciam.rsc03.net/servlet/cc?lJpDVUWEmLtisHklLkpLlFHhsDJhtE0EV
 |
12:47 17 June 03 NewScientist.com news service A computer memory chip based on carbon nanotubes has passed a manufacturing milestone, according to the US company developing the technology. The prototype chip would store information using hundreds of billions of nanotubes with a theoretical capacity of 10 gigabits of data, says Nantero, based in Boston, Massachusetts. Once fully developed, the company says nanoscale random access memory (NRAM) could hold more data that existing types of RAM and would also be non-volatile, meaning data would not be lost when the power is been turned off. Computers using such memory could boot up almost instantly. Nantero also claims that NRAM would be much faster than current non-volatile memory, such as Flash. Nantero is not the only company hoping to use carbon nanotubes to make improved types of computer memory. But the company believes its advantage lies in the fact that its chips can be made using existing silicon manufacturing methods and would therefore be relatively cheap to make. Random arrangement Instead of trying to grow nanotubes in the correct alignment, Nantero applies them randomly across the entire surface of a silicon wafer. It then uses existing lithographic equipment to etch away the nanotubes that are not in the correct alignment. "The creative breakthrough is to put nanotubes everywhere," Nantero's CEO Greg Schmergel told New Scientist. The nanotubes remaining after etching are arranged in bunches across pairs of electrodes on the surface of the wafer. Applying a small electrical field alters the tubes so that they either bridge the gap between the electrodes or do not. These two states result in different conductivity that is easy to detect and can be used to represent a binary one or zero. Nantero has now produced a wafer dotted with nanotube clumps, but is still developing the way of addressing each individual bunch. Schmergel says this is just a matter of harnessing existing silicon electronics technology. Cees Dekker, an expert in carbon nanotubes at Delft University in the Netherlands, says the fabrication technique appears workable. But he says a potential problem lies in the difficulty of separating the different types of nanotubes that are created together during their creation. "You have to find a way to deal with both semiconducting- and metallic-type nanotubes, which have rather different electrical properties," he told New Scientist. Schmergel expects to have NRAM memory capable of storing up to four megabits in 18 months and components that could compete with current types of RAM in around three years. Will Knight http://www.newscientist.com/news/news.jsp?id=ns99993838
|