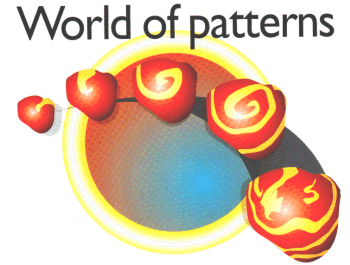
 |
| Figure 1: If part of the heart is damaged during a heart attack, it can act as an organising centre, making the muscle cells contract in rotating spiral waves. If a wave breaks apart (far right), the result can be fatal |
Patterns are everywhere
in nature.By finding the connections between them,physicists
can begin to understand the mysterious forces that draw spirals
spots and stripes where before there were none.
Mark Buchanan
WE'VE all been there. Rolling down the motorway, the traffic grinds to a sudden halt. For the next hour you creep along at walking pace, peering ahead to see the road works or accident that caused the hold-up. But there isn't one, and finally the traffic clears as if by magic, and you're off again.
Phantom blockages like this seem wholly inexplicable, especially from ground level. But if you had a bird's-eye view, you'd see them for what they really are-a pattern that forms simply because there are too many cars. As the traffic increases, cars begin to pack closer together. Drivers with less room to manoeuvre touch their brakes more frequently. Ultimately, when the density of cars becomes too high, the situation goes unstable. In a natural process known as spontaneous pattern formation, clumps of traffic develop and pretty soon pile-ups emerge every few kilometres.
When patterns like these appear on the highway, they can be irritating or perhaps even dangerous. But to physicists and mathematicians they have a more fundamental significance. The same spontaneous process that clogs up the traffic also paints spots on a leopard's back and stripes on a zebra, dots the sky with wispy clouds, and carves out the jagged patterns of cracks in a broken brick or piece of glass. It steers the powerful ocean currents that influence the weather, and fixes the peculiar structure of every snowflake as it grows. It creates dangerous waves that can upset a regular heartbeat (see Figure 1). Spirals in shells, ripples in sand dunes-in every case, they emerge from a medium that starts out uniform and featureless. Then something happens, and a pattern forms.
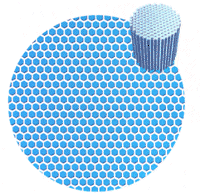 |
| Figure 2: Heating a shallow layer of liquid from below can create a pattern of hexagonal cells viewed from above. Warm liquid flows up in the centres of the hexagons and down at the boundaries between them (see top right) |
But which pattern forms under which conditions? And why do they end up looking the way they do? For centuries, scientists and engineers have been collecting and describing nature's patterns, and building a theory to explain how they come to be. In the past few decades, they have made enormous progress. The theory of pattern formation can now account pretty well for the patterns that appear in everything from river networks to bacterial colonies-not to mention traffic on the motorway.
The French physicist Henri Bénard's discovered one of the simplest and richest examples of pattern formation in 1900, when he was experimenting with a shallow layer of liquid that he heated from below. Bénard found that when the heating was weak, the liquid remained motionless. Energy moved up through it by conduction, each layer passing warmth to the one above it. But as Bénard turned up the heat, he reached a point where the liquid suddenly burst into motion. Now, parcels of moving fluid carried heat energy upwards. These parcels formed into a network of convection cells, which when viewed from above showed up as a pattern of hexagons (see Figure 2). In each cell, warm liquid was flowing upwards in the dark centre, and cooler liquid was sinking at the boundaries.
Bénard didn't know how to explain these findings, but a few years later the British physicist Lord Rayleigh thought he did. He realised that the important question was not why the liquid suddenly started to convect but why it hadn't been convecting all along. Near the bottom of the container, where the heat is applied, the warmer liquid expands and becomes less dense than the cooler liquid above. The resulting buoyancy forces should therefore cause the warm liquid to rise, while the cooler, denser liquid sinks, setting the process of convection in motion.
Why doesn't this happen the moment you start heating the liquid? It would, Rayleigh reasoned, if the liquid didn't have a kind of internal friction-known as 'viscosity-that retards its motion. If the heating is too weak, viscosity prevents the warm liquid rising and cold liquid sinking, and convection never begins. With more vigorous heating, the buoyancy forces grow and eventually become strong enough to set the fluid into motion. When that happens, the fluid must go up in some places and down in others, and Rayleigh showed that this would produce a regular pattern, just as Bénard had observed. We now it now that Rayleigh's explanation left out one important detail (see Box 1). But it came close enough to illustrate some general principles at work in pattern-forming systems of all kinds.
The first principle is that patterns only emerge when a system crosses a
stability threshold: it has to be driven sufficiently far from equilibrium
before a pattern starts to appear. Take Bénard 's experiment. At first,
when the heating is weak and the water is close to equilibrium, any small
disturbance will stir up forces that quickly bring the liquid hack to the
motionless state. It acts like a ball resting at the bottom of a bowl. But
then, as the heating becomes stronger, there comes a point beyond which the
forces triggered by a disturbance will only make it grow larger. Past this
threshold, even the microscopic random noise of
molecular motion is enough to tear the
uniformity apart. The stage is then set for a pattern to emerge.
Breaking the mould
Perfect patterns
THE SECOND principle illustrated by the convection experiment is that when patterns form, they do so through a process known as spontaneous symmetry breaking. The liquid originally has high symmetry. Every spot is like every other; there is no pattern. The force applied to the system also has high symmetry, as it is being heated uniformly. You would anticipate that a uniform force on a uniform system would lead naturally to a uniform effect- but it doesn't. The emergence of the pattern shatters the perfect symmetry. In Bénard's experiment, all points in the liquid were originally equivalent. Once the hexagons form the liquid moves upwards at some points and downwards at others. Now the liquid is no longer perfectly symmetrical, because not all points are the same.
| 1: Kinds of convection WHEN Lord Rayleigh developed his theory to explain convection patterns, he must have been dismayed to find that it didn't entirely agree with Henri Bénard's observations. But the discrepancy is understandable, as the two scientists were considering what turn out to he completely different problems. Bénard's held his liquid in an open container, with the liquid's surface exposed to the air. Rayleigh's theoretical fluid totally filled a closed container leaving no exposed surface. This may seem an insignificant difference, but it meant that there was an additional force at work in Bénard's experiment that Rayleigh's calculations did not take into account. That force is surface tension. In a liquid, molecules form weak bonds with their neighbours wherever possible. A molecule buried inside the liquid can form bonds with molecules on all sides. But molecules at the surface aren't so lucky. Unable to form bonds on one side, these surface dwellers have higher energy than their buried cousins. The extra cost in energy of an exposed surface causes a natural tension, which gives it a tendency to shrink to as small an area as possible. This is why water, for example, forms into spherical droplets, which have the smallest possible surface area for a given volume. In Bénard's experimental setup, the liquid cannot shrink. But it can do something else. The surface tension of a liquid decreases with increasing temperature, so it can reduce the surface tension by bringing up warm liquid from the depths. In Bénard's experiment, this is actually the dominant process, which is why Rayleigh's calculations didn't workout. Since then, physicists have investigated Rayleigh's setup and found that his calculation was spot on. |
In every case where patterns form, these two principles are found to be at work. But there is a third important principle about patterns, too: similar patterns often pop up under wildly different conditions-even in completely different systems. It turns out, for example, that Bénard wasn't the first to see geometric patterns standing out in a liquid. That honour goes to Michael Faraday, who in 1831 discovered that simply by vibrating a thin layer of liquid he could make standing waves form. As always, there is a threshold. If the vibration is slow, the liquid remains in a uniform layer. More vigorous vibration destroys the uniformity and breaks the symmetry: ridges and troughs appear on the surface of the liquid, forming stripes or chessboard patterns.
In a similar way, patterns can also be made to appear in a layer of ordinary sand in an empty box, just by shaking it. Physicist Harry Swinney and his colleagues at the University of Texas at Austin have found that this simple activity produces an astonishing spectrum of patterns. When the shaking is vigorous enough, the flat layer of sand suddenly starts piling up. Sometimes it forms a striped pattern of ridges and valleys, sometimes ridges that link up to form networks of squares or hexagons.
Over the past decade, Swinney's team has mapped out the startling range of patterns that form for different frequencies and amplitudes of shaking. The researchers have learnt that they can tune the shaking to move the sand from one pattern to another, "melting" a grid of hexagons, for instance, into a row of stripes.
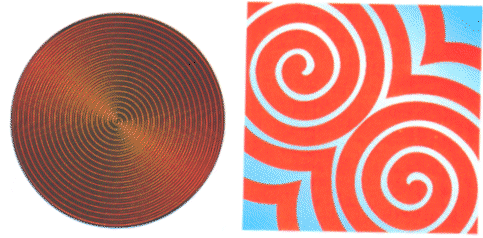
But even the rich variety of patterns in shaken sand only hints at the diversity of the patterns that is possible in systems driven far from equilibrium. When liquids are heated, the convection rolls in some cases forgo the hexagonal arrangement, and instead grow into peculiar rotating spirals (see Figure 3). Similar spirals also show up in chemical reactions.
A classic example is the
Belousov-zhabotinsky reaction,
named after Russian chemists Boris Belousov and Anatol Zhabotinsky. This
is an example of a reaction-diffusion system. The name refers to the two
general forces at work in it. Reactions occurring at a particular point cause
the concentrations of chemicals there to change, becoming higher or lower
than they are at points nearby. At the same time, random thermal motion of
molecules tends to degrade these differences by the process of diffusion.
In essence, reactions build differences up, while diffusion washes them out.
Patterns emerge out of the balance.
Stripes and spots
Animal magic
IN the 1950s, the British mathematician Alan Turing speculated that this basic process might underlie the way patterns appear in living things-including how animal embryos develop arms, legs, fins and their beautiful coloured markings. All organisms are, after all, complicated chemical systems. Turing showed that systems described by a general class of reaction-diffusion equations would form static patterns, now known as Turing patterns, which bear a remarkable resemblance to the colouring patterns of animals such as leopards or tigers. Turing patterns can be seen in the Belousov-Zhabotinsky reaction. But the cellular chemistry that leads to skin colouring is generally quite complicated, so it may be a while before we can make a precise connection. Understanding the development of arms and legs may also follow, although in this case Turing patterns tell only a small part of the story.
| 2: Dendrites THE SNOWFLAKE may be the best-known example of a beautiful, many-branching pattern, but it is far from being the only one. Take molten metal, for instance, cooled to just below its freezing point. The metal will begin to solidify along a front, And the growing front moves into the liquid as it gradually gobbles up one molecule after another. It would like to grow as fast as possible, but finds itself up against a natural braking mechanism. Each time a molecule from the liquid attaches itself to the solid front, its energy decreases. That little bit of energy has so go somewhere: it appears as heat that diffuses away. So the front can only grow as quickly as diffusion can carry the heat away. This is where instability comes into play. If, for whatever reason, a tiny bump forms on the growing surface, this alters how fast heat can flow away. The details are rather involved, but the conclusion is simple: the growth rate at this point increases slightly and the hump begins to grow faster than anything else on the front. So the solid does not grow into a regular lump, but some wilder thing decorated with branches that have themselves sprouted branches. Do this as many times as you like, and the shape you get will always be unique (see Figure 5). |
A whole new family of patterns appears in a class of systems known as excitable media. Heart tissue is a good example of such a medium. The muscle cells in the heart contract when they are excited by an electrical impulse. As the impulse travels through the tissue it causes it to contract in an organised way. Generally this is just what is needed: it is how your heart beats, and pumps blood through your body. But if a small region of the heart becomes damaged-in a heart attack, for instance-it can act as an organising centre, directing waves of excitation to travel in closed loops, yielding rotating spiral waves like the ones seen in chemical reactions (Figure 1). These can be deadly, so researchers are working on ways to control these spirals and drive them out of the heart.
If you get the feeling that there are as many patterns as there are systems, you are not far wrong. Nevertheless, physicists have discovered the elements of a general theory that covers them all. In many cases, it turns out, patterns result from a basic competition between a set of forces that tends to amplify differences, and another that tends to dampen them out-as in the reaction-diffusion system. As it turns out, the details of this or that system are not important. No matter what specific forces lead to nonuniformity on the one hand or uniformity on the other, the battle between them often plays itself out in the same way.
To highlight this crucial battle, without the distraction of detailed information
about the forces that are at work, physicists rely on a powerful concept
known as the order parameter, which was invented in the 1940s by the
great Soviet physicist Lev Landau. For a layer of sand being shaken in a
box, the order parameter might be the layer's average height relative to
its undisturbed state. Below the pattern-forming threshold, this order parameter
is zero- there is no pattern. Above the threshold, the order parameter follows
a mathematical function that reflects the pattern in the sand: it takes on
a positive value where the sand is high, and a negative one where it is low.
For liquid convection, the order parameter would be different: it might be
the horizontal component of the liquid's velocity at the surface. Again,
this is zero in the uniform state, and otherwise takes on the form of the
emerging convection rolls.
| Figure 3: When liquids are heated, they sometimes forgo the hexagonal pattern shown in Figure 2, and develop rotating spirals instead (left). Similar spirals show up in chemical systems such as the Belousov-Zhabotinsky reaction (right) |
 |
By thinking in terms of order parameters, scientists can leave aside the ugly details of sand or liquid or whatever other substance might be involved. Just by knowing the basic forms of the forces involved they can derive an equation for the order parameter that captures the broad picture of how the battle between uniformity and nonuniformity should play itself out. Fortunately, it seems that there are only a few basic categories of battle-corresponding to a few basic kinds of forces. As a result there is also just a handful of pattern formation equations, each of which applies to a wide class of physical systems.
One of these equations, known as the Ginzburg-Landau equation, describes the way patterns form in chemical systems such as the Belousov-Zhabotinsky reaction. It also describes how patterns arise in superconducting materials when they are driven out of equilibrium, and to pattern formation in laser systems, in heart muscle and in colonies of amoebas.
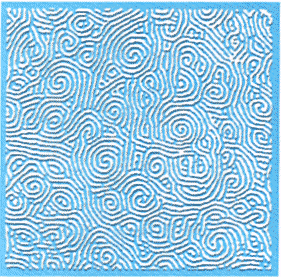
| Figure 4: When a fluid is heated vigorously, it can form many tiny spirals, which mingle with one another to create a pattern called "spiral defect chaos" |
 |
The fact that a single equation applies to such superficially different systems implies that the patterns arise not by virtue of the particular properties of sand, water, chemicals or tissues but through universal principles that work the same way in every setting. They are deep phenomena, more a consequence of mathematics than of physics.
Some physical systems produce much more complex patterns-so complex, in fact, that you never see quite the same pat tern twice over. Think of snowflakes. They grow when ice crystallises from water vapour in the atmosphere, and the process always leads to patterns with similar basic characteristics: every snowflake has the symmetry of a hexagon. But if you look at a snowflake more closely you will see that it also has an intricate pattern of side branches sprouting from the main body. The complicated pattern of branching ensures that no two snowflakes are alike.
Johannes Kepler tried to explain these shapes in 1611. How, he wondered, can such beautiful patterns arise without any guiding hand from some designer? Nearly four centuries later, physicists still don't know how to describe the growth of real snowflakes in detail, because the properties of water are rather complicated. But the basic principles have become clear. Snowflakes typify a class of patterns that form by a process called dendritic growth. The same process underlies how ice grows on a lake in winter, or metal solidifies from a melt during the formation of a weld (see Box 2). Crystals of ice and metal grow one molecule at a time, as particles diffuse in from the surrounding liquid to attach themselves to the solid. Physicists have developed a class of mathematical models to describe this process, which they call diffusion-limited aggregation (DLA)-an ugly term for a beautiful process which generates spectacular patterns.
It works like this. You start with a single molecule, and let another approach it from a random direction, following a random path. The second molecule can either run into the first and stick, or miss and wander off. The idea is to repeat the process millions of times and see what happens. You might expect that this process would make the cluster grow into an uninteresting blob, but if you look a little deeper you can see why it doesn't. Once the structure has developed its branched arms, it becomes very unlikely that a molecule can make it in towards the inside. So it almost always gets attached near the end of one of the longer arms, making it still longer. This structure is a fractal, which means that its pieces look very much like the whole.
| Figure 5: Whether it is water or metal that solidifies to create beautiful patterns like this one, the processes involved are so complicated that no two examples are alike. |
 |
The many-fingered DLA patterns often show up when a thin film of one material
is deposited on another, or when one fluid displaces another-a process known
as viscous fingering. Think of
two glass plates with a
thick, viscous liquid trapped in between. Now bore a tiny hole in one
of the plates and force air slowly into the liquid. As air pushes the liquid
out of the way, it doesn't do so in a geometrically simple way. Instead,
it grows fingers, and the fingers grow fingers. The resulting pattern has
mathematical properties typical of fingered DLA patterns. Similar patterns
have been seen in many other situations, including the distributions of people
in cities.
As with the Ginzburg-Landau equation, the DEA process arises in many different
systems.The further scientists explore, the more patterns they encounter.
Even convection still holds mysteries. Though the hexagonal patterns that
form just above the stability threshold are simple enough, they become
increasingly complicated as the fluid is heated more vigorously. One common
pattern occurs when the convection spontaneously generates tiny spirals,
which mingle with one another throughout the container. Physicists refer
to the discontinuous regions where the spirals meet as "defects", and to
the churning state containing many broken spirals as spiral defect chaos
(see Figure 4).
Researchers are still trying to understand these patterns. They hope that by getting to grips with spiral defect chaos they may come up with clues for understanding the most complex pattern of all fluid turbulence, the wild and erratic motion that occurs in every raging stream. Turbulence seems to be the spiritual cousin of spiral defect chaos. And, though the complexities of turbulence have so far defeated physicists, they do have some universal aspects. If you stir any liquid or gas vigorously enough, the swirling maelstrom you get is, in its statistical character, always the same. The universal state of turbulence lies at the very edge of the world of patterns. But by hopping from one pattern to another, taking one small step at a time, physicists should one day get there.
© Reed Business Information 1999. Educational copying
of Inside Science is permitted under licence of Copyright Licensing Agency
Limited, 33 - 34 Alfred Place, London WC1 7DP
Back numbers of Inside Science are available
from New Scientist, P.O. Box 666, London ElS 1DW. Inquiries 0181
5030589.